CAPA Practitioner for Medical Devices Training
CAPA Practitioner for Medical Devices Training that covers CAPA requirements, common root cause investigation tools, and how to prepare for an inspection of the CAPA process. Our training is for the MedTech Industry including those involved in any part of a CAPA system.
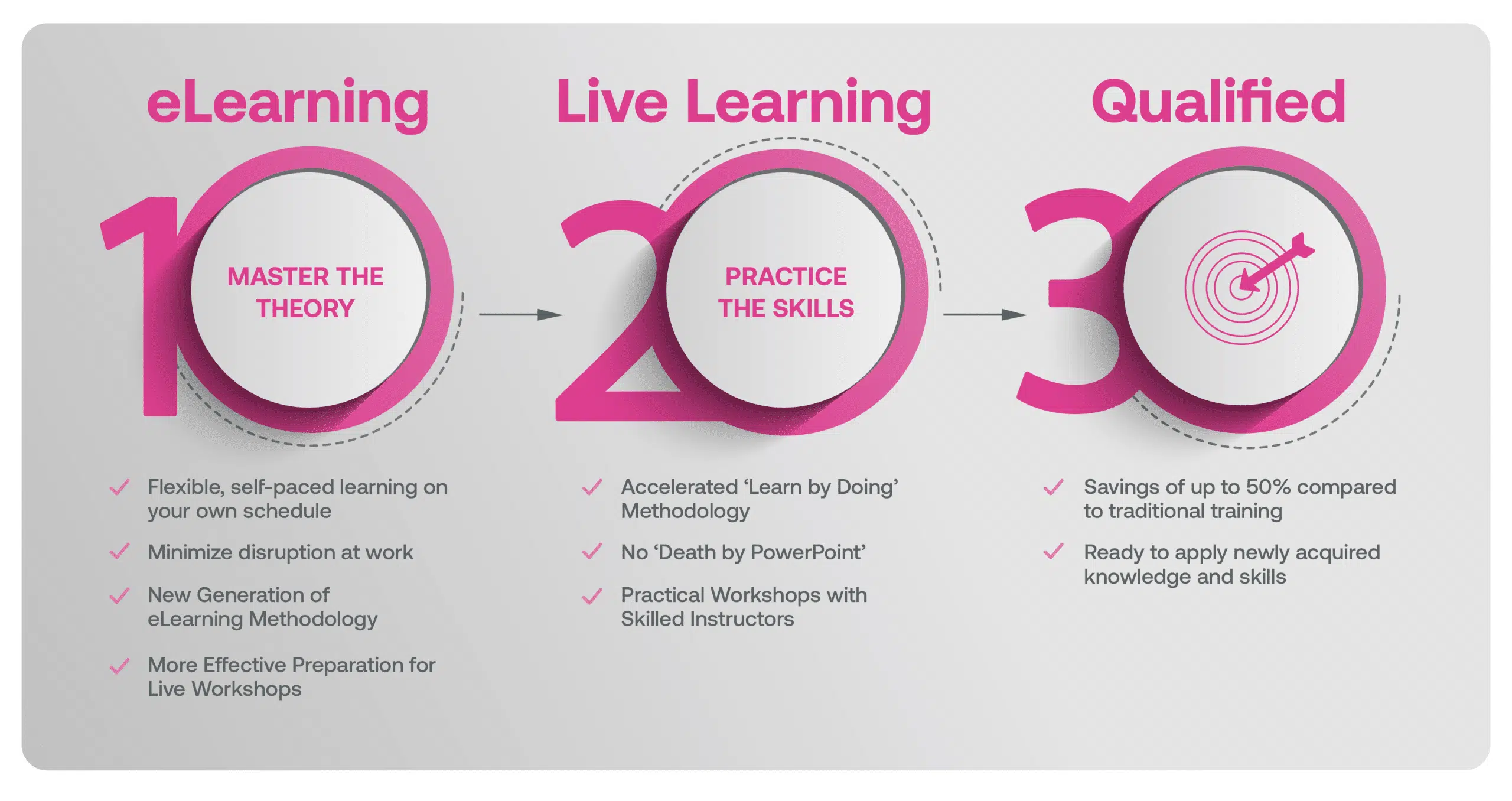
Our blended learning course is a hybrid approach where you first complete 6hrs of eLearning modules on the underlying theory that better prepares you in advance of attending a 1-day Live Workshop with an Instructor that focus on practical application in a real life context.
This course recognized by the Regulatory Affairs Professionals Society (RAPS) offering global recognition for successful learners. RAPS members will also be eligible to receive 12 credits by completing this course.





View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain a Recognized CAPA Practitioner Qualification
Advance your knowledge and upskill with one of our Instructors.

Register 3 + Get 1 Free

Course Overview
CAPA Training for Medical Devices
At the end of this course, Learners will have the knowledge & skills to:
- Understand & implement the Seven CAPA Steps approach.
- Understand & meet FDA and European expectations when completing CAPAs.
- Learn how to conduct thorough Investigations into the causes of failure.
- Identify between Correction and Corrective Action.
- Identify between Corrective Action and Preventive Action.
- Generate Corrective Actions that address the Root Causes of failure.
- Document SMART Effectiveness Checks criteria.
- Determine the Effectiveness of Corrective Actions.
- Determine the Overall Effectiveness of a CAPA system.
This course would be targeted at those within the MedTech Industry who are involved in any part of a CAPA system, including:
- Operators
- Technical Staff
- Managers
- Quality/Regulatory Affairs Personnel
- Engineering or Support Functions
Before completing this course, each Learner should have the following knowledge and/or experience:
ISO 13485
- Must have experience of working with ISO 13485:2016 or knowledge of ISO 13485:2016
Medical Device Quality Management Systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices
- The process approach used in quality management
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (12).
Successful completion will entitle each Learner to receive a digital Certificate of Achievement within 1 business day.
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based) by the required deadline set in advance of the given workshop dates you are registered for (applies to blended format only)
- Fully attend the Instructor Workshops as 100% attendance is required
- Obtain 70% or higher in the graded assessments during the Instructor Workshops
If you are completing this training virtually, the following applies:
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
Blended Learning is Better Learning
A two-step methodology with eLearning modules that help you master the theory better preparing you for practical workshops that embed the skills.
Course Structure Explained
Detailed Breakdown & Agenda
Learners first complete interactive eLearning modules to grasp the underlying theory, then attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| Module 1 | Introduction to CAPA
Case Study A medical device failure and the need for CAPA |
| Module 2 | Corrective Action – Fundamentals
Case Study Conduct a root cause analysis using provided data |
| Module 3 | Preventive Action – Fundamentals
Case Study Proactive Risk Mitigation |
| Module 4 | Integrating CAPA with the QMS
Case Study Identifying gaps in a CAPA process |
| Module 5 | CAPA Process Verification and Monitoring
Case Study Auditing CAPA effectiveness |
| Time | Topic |
|---|---|
| #1 | eLearning Recap Practical Workshop Identifying the Problem (Problem Statement – Scenarios), continued. |
| #2 | Practical Workshop Evaluating the Risk and Impact, Determining the Plan to Investigate and Investigating the Problem and determining its Cause Practical Workshop Create an Action Plan and Implement CAPA Action Plan |
| #3 | Practical Workshop Verify Effectiveness and CAPA Process Steps |
| #4 | RCA Methodologies Introduction Practical Workshop 5 Whys, 8D Root Cause Analysis, Cause & Effect, Pareto Chart |
| #5 | Practical Workshop Root Cause Analysis (5 whys, Fishbone, 8D) |
| Q&A | Course Survey and Close |
Our Experts
Meet The Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About our CAPA Training
Learn about the benefits and key features of Comply Guru’s Blended Learning.
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.5
Average Rating
89 global ratings
-
The workshop was engaging and highly interactive. Presenter had lots of practical experience, was very approachable and welcomed questions.
-
Very enjoyable training.
-
Michelle is a great trainer and made us all feel extremely comfortable and a safe space to discuss things with confidence
-
Michelle is great trainer and shared great information that helped me to improve our CAPA process
-
Great informative content, good mix of individual and delivered.