MDSAP Auditor Skills Training that provides learners with the knowledge and skills required to understand and complete an audit against the IMDRF Medical Device Single Audit Program (MDSAP) for medical device quality management based on ISO 13485 and international regulatory bodies medical device regulations.
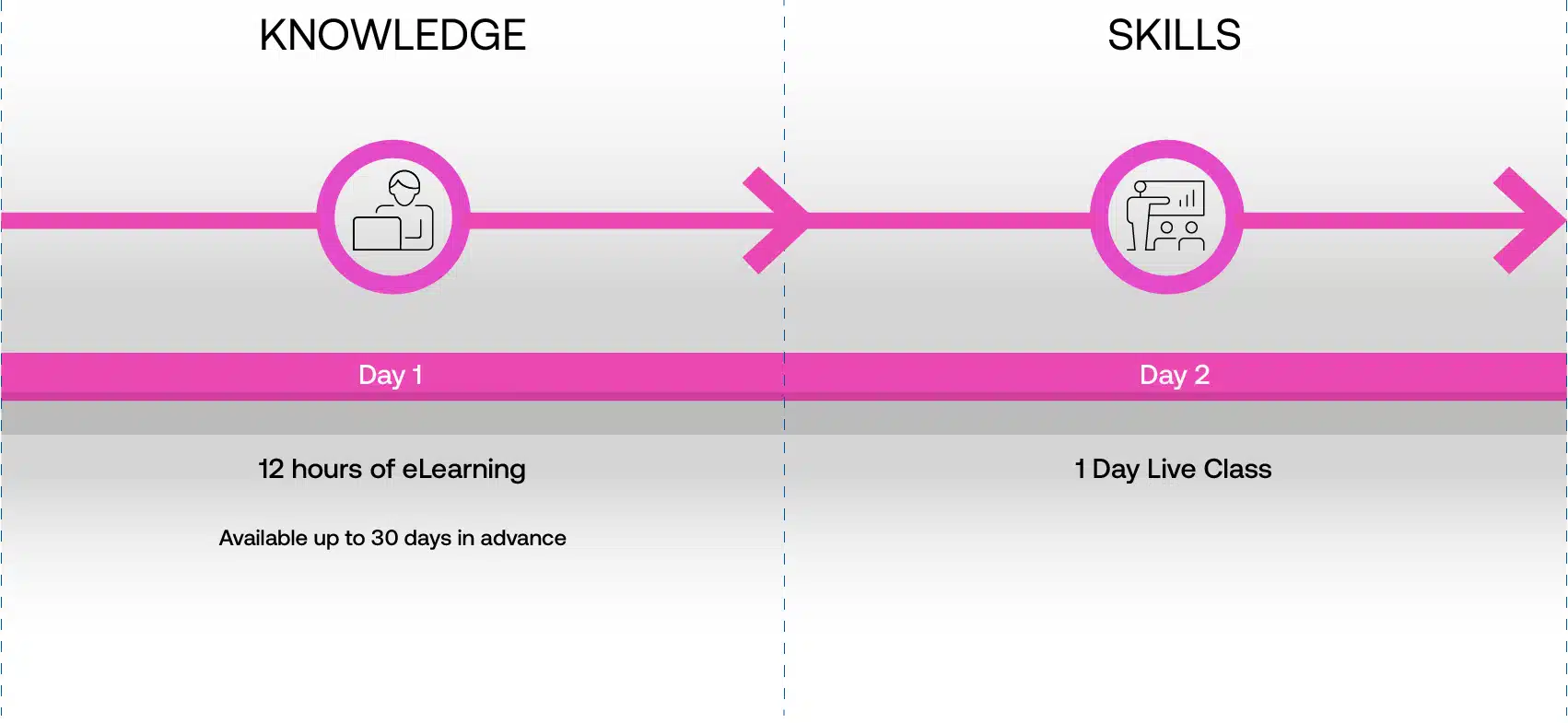
Our Blended Learning Methodology is a hybrid of 8hrs of highly interactive eLearning theory modules that are completed first before attending a 1-day skills-based Live Instructor Workshops that focuses on practical application in a real life context.
Upcoming Schedule
Gain an Accredited MDSAP Auditor Skills Qualification
Advance your knowledge and enjoy more flexibility & learning effectiveness with blended learning.

Our Methodology
How Blended Learning Works
Blended learning is a two-step approach: Beginning with eLearning modules to grasp the foundational theory, then attending live, instructor-led classes emphasizing practical, real-world application.
Course Structure Explained
Detailed Breakdown & Agenda
Learners first complete interactive eLearning modules to grasp the underlying theory, then attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| 35mins eLearning | Module 1 – Introduction to MDSAP
|
| 125mins eLearning | Module 2 – MDSAP Audits
Case Study! Role of the Australian Sponsor Case Study! Audit Time Determination |
| 95mins eLearning | Module 3 – MDSAP Chapters 1 and 2
Case Study! Management Responsibility Case Study! Purchasing Controls |
| 110mins eLearning | Module 4 – MDSAP Chapters 3 and 4
Case Study! Data Sources, Preventative Action, Verification of Effectiveness and Management Representative Case Study! Process Change and Regulatory Impact Case Study! Adverse Event Reporting |
| 95mins eLearning | Module 5 – Design and Development
Case Study! Outsourced Design and Development Case Study! Software and Hardware Change |
| 125mins eLearning | Module 6 – Production and Service Controls
Case Study! Training and Documentation Case Study! Medical Device File Case Study! Control of Cleanliness of Product Case Study! Software Validation |
| 95mins eLearning | Module 7 – MDSAP Chapter 7 and Annexes
Case Study! Technical Documentation Case Study! Customer vs Supplier vs Outsourcing |
| 45mins | eLearning Assessment (45mins)
|
| Time | Topic |
|---|---|
| 08:30 - 9:30am | Course Introduction |
| 9:30 - 9:40am | Break |
| 9:40 - 10:45am | Practical Workshop Audit Plan, Continued |
| 10:45 - 11:00am | Break |
| 11:00am - 12:00pm | Practical Workshop Audit Checklist (Continued) |
| 12:00 - 12:10pm | Break |
| 12:10 - 1:00pm | Practical Workshop Opening Meeting |
| 1:00 - 1:45pm | Lunch |
| 1:45 - 2:45pm | Practical Workshop Generating Audit Findings |
| 2:45 - 3:00pm | Break |
| 3:00 - 4:00pm | Practical Workshop Generating Audit Findings (Continued) |
| 4:00 - 4:10pm | Break |
| 4:10 - 5:10pm | Practical Workshop Closing Meeting, Audit Report & Follow-Up |
| 5:10 - 5:15pm | Break |
| 5:15 - 5:45pm |
|
Course Overview
MDSAP Auditor Skills Training
Explain the purpose of a medical device single audit program (MDSAP) medical device quality management system audit process in the context of ISO 13485 and regulatory compliance.
Explain the role and responsibilities of an auditor to plan, conduct, and report nonconformities for a quality management system audit in accordance with ISO 17021, regulatory body requirements and the MDSAP audit criteria.
Plan for and conduct an audit of a medical device quality management system, report, and grade non-conformities to establish compliance with ISO 13485 and regulatory requirements using MDSAP methodology.
- Quality Assurance Professionals
- Quality Engineers
- Regulatory Professionals
- Internal Auditors
- Lead Auditors
CQI & IRCA certify this course (No. 2694). Upon successful completion, each Learner shall receive a digital Certificate of Achievement.
Prior to attending this course, learners are expected to have the following prior knowledge:
ISO 13485
- Knowledge of the ISO 13485 Requirements
- Must have successfully completed a CQI and IRCA Certified MD-QMS ISO 13485 Internal or Lead Auditor course or equivalent.
Medical device management systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
- Commonly used quality management terms and definitions within ISO 13485 and ISO 9000.
- The process approach used in quality management.
- A working knowledge of risk-management principles related to the design of a medical device, through ISO 14971.
To successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based) by the required deadline set in advance of the given workshop dates you are registered for.
- Fully attend the Instructor Workshops as 100% attendance is required.
- Obtain 70% or higher in the graded assessments during the Instructor Workshops
For the live workshops during a virtual delivery, we utilise both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Why Choose Comply Guru
Why Choose Comply Guru's Blended Learning?
Learn about how our Blended Learning Methodology is leading the industry for innovation & learning effectiveness
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.8
Average Rating
32 global ratings
-
It was great to work with CoomplyGuru for this training. The support was very good all throughout the training. The trainer was very accommodating. The flexibility of the training was very much appreciated as some of the attendees were supporting multiple site audits during a portion of the course.
-
Trainer was great, very knowledgeable and good at maintaining the pace and interest in the material, the online section presented an opportunity to get simpler details out of the way which enhanced the classroom experience.
-
Great approach on MDSAP auditing, John is a great instructor, very dynamic and giving concrete examples.
-
It was excellent, I found the pre course training excellent and on the day the tutor had many open discussion with lots of interaction
-
The course met my needs, I found the blended learning a good learning technique.
Frequently Asked Questions






