EU MDR Lead Auditor Training for the European Medical Device Regulation (2017/745)
EU MDR Lead Auditor Training to provide learners with the knowledge and skills required to lead audits of Medical Device Quality Management Systems (MD-QMS) against the European Union’s Medical Device Regulation (EU MDR 2017/745) in accordance with ISO 19011 and ISO 17021, as applicable.
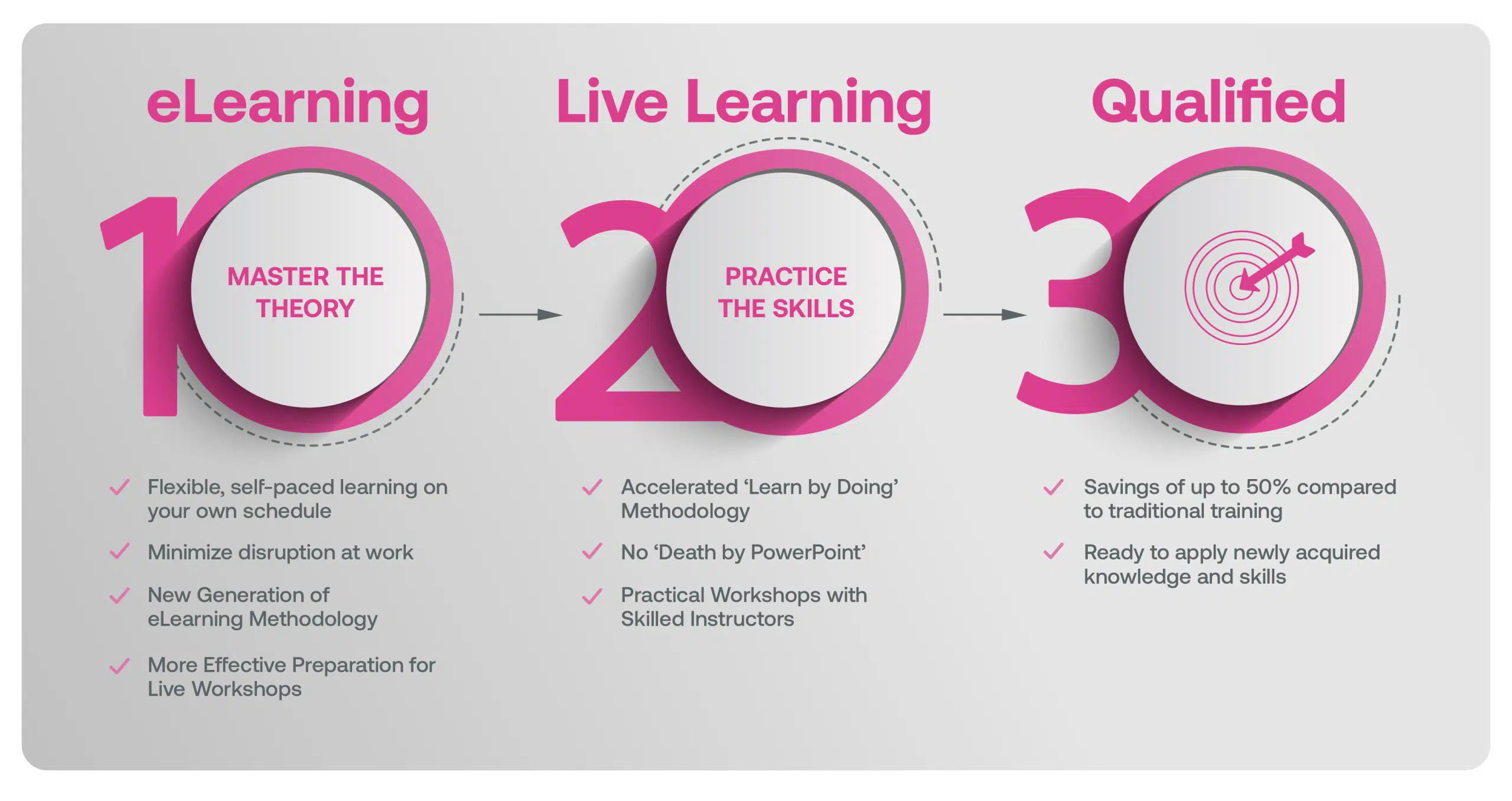
Our blended learning course is a hybrid approach where learners first complete eLearning modules on the underlying theory that better prepares learners in advance of attending Live Classes with an experienced Instructor. Our live workshops are based on practical training methods with real-world examples, case studies, facilitated exercises and group discussions.
CQI and IRCA certify this course (No. 2696) offering global recognition for successful participants.





View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain a Globally Recognized MDR Lead Auditor Qualification
Advance your knowledge and enjoy more flexibility & learning effectiveness with Comply Guru.

Register 3 + Get 1 Free

Course Overview
EU MDR Lead Auditor Training
Explain the background and purpose of a medical device quality management system (MD-QMS) in the context of EU MDR 2017/745 and confirm links with ISO 13485.
Explain the role and responsibilities of an auditor to plan, conduct, and report nonconformities for a quality management system audit in accordance with ISO 19011/ISO 17021 that uses the EU MDR 2017/745 requirements as the audit criteria.
Plan for and conduct an audit of a medical device quality management system to establish compliance with the EU MDR 2017/745 in accordance with ISO 19011/ISO 17021 as applicable and report on any nonconformities.
CQI & IRCA certify this course (No. 2696). Upon successful completion, each Learner shall receive a digital Certificate of Achievement.
Prior to attending this course, learners are expected to have the following prior knowledge:
EU MDR
- A good understanding of the EU MDR (2017/745) requirements and their application is required for this course. This may be gained by successfully completing CQI and IRCA Certified MD-QMS Comprehensive EU-MDR 2017/745 Practitioner (PT219) course. A copy of the training certificate will be requested for verification purposes as part of acceptance onto the course.
Please note: if no recognized certificate is provided, additional information & a pre-course test may be required to verify knowledge of the requirements in order to be accepted onto the course.
ISO 13485
- Must have successfully completed a CQI and IRCA Certified MD-QMS ISO 13485 Lead Auditor course or equivalent. A copy of the training certificate will be requested for verification purposes as part of acceptance onto the course.
Medical device management systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
- The process approach used in quality management.
- A working knowledge of risk-management principles related to the design of a medical device, through ISO 14971.
To successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based) by the required deadline set in advance of the given workshop dates you are registered for.
- Fully attend the Instructor Workshops as 100% attendance is required.
- Obtain 70% or higher in the graded assessments during the Instructor Workshops
- Pass the end of course final assessment.
For the live workshops during a virtual delivery, we utilise both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
Blended Learning is Better Learning
A two-step methodology with eLearning modules that help you master the theory better preparing you for practical workshops that embed the skills.
Course Structure Explained
Detailed Breakdown & Agenda
Learners first complete interactive eLearning modules to grasp the underlying theory, then attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| 90mins eLearning | Module 1: Introduction to the EU MDR
|
| 120mins eLearning | Module 2: Placing a Device on the Market
|
| 90mins eLearning | Module 3: Post Market Surveillance and Vigilance
|
| 120mins eLearning | Module 4: Technical Documentation, UDI and EUDAMED
|
| 60mins eLearning | Module 5: EU MDR Auditing
|
| 40mins | eLearning Exam
|
| Time | Topic |
|---|---|
| 08:00am | Course Introduction
Practical Workshop Mapping the Regulatory Pathway |
| 09:00am | Break |
| 09:15am | Medical Device Classification
|
| 10:15am | Break |
| 10:30am | MDR Conformity Assessment Routes
Practical Workshop Medical Device Conformity Assessment Routes |
| 11:30am | Break |
| 11:45am | Audit Lifecycle under the EU MDR 2017/745
Practical Workshop Audit Lifecycle |
| 12:30pm | Close |
| Time | Topic |
|---|---|
| 08:00am | Simulated MDR Audit – Execution Phase
Practical Workshop Simulated MDR Audit |
| 09:00am | Break |
| 09:15am | Simulated MDR Audit – Execution Phase (Cont’d)
Practical Workshop Simulated MDR Audit |
| 10:15am | Break |
| 10:30am | Simulated MDR Audit – Execution Phase (Cont’d)
Practical Workshop Simulated MDR Audit |
| 11:30am | Lunch |
| 11:45am | Assessment Review / CQI-IRCA Specimen Exam
|
| 12:30pm | Close |
| Time | Topic |
|---|---|
| 08:00am | Assessment Review
|
| 09:00am | Break |
| 09:15am | Reporting Audit Findings
Practical Workshop Audit Findings |
| 10:15am | Break |
| 10:30am | Reporting Audit Findings
Practical Workshop Audit Findings |
| 11:30am | Break |
| 11:45am | Audit Report
Practical Workshop Audit Reporting |
| 12:30pm | Close |
| Time | Topic |
|---|---|
| 08:00am | Closing Meeting
Practical Workshop Closing Meeting Role Play |
| 09:00am | Break |
| 09:15am | Audit Follow-up Activities
Practical Workshop Follow Up |
| 10:15am | Break |
| 10:30am | Final CQI & IRCA Exam Certification Exam (via eAssessment) |
| 12:30pm | Close |
Our Experts
Meet the Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About Our Lead Auditor Training
Learn about how our Blended Learning Methodology is leading the industry for innovation & learning effectiveness
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.9
Average Rating
17 global ratings
-
Informative and interactive
-
Very well structured online content with exchange between theory and testing at optimal intervals +final tests. The instructor of the tutor-based part is very knowledgeable and with great experience from the field.
-
Overall I think this course was excellent.
-
Very engaging, comprehensive detail on topic, great interactive class discussion, flexible but also encouraged full participation by all involved.
-
It has met my expectations. Thank you
Frequently Asked Questions