EU MDR Requirements Training for Practitioners working with the European Medical Device Regulation (2017/745)
EU MDR Requirements Training for Practitioners that takes you through the new European Medical Device Regulation (2017/745), not just from an industry perspective, but also from a Notified Body perspective. This course has been specifically designed for Quality & Regulatory Professionals who are new to the Regulation.
What makes Comply Guru’s eLearning different from competitors is we instructionally design modules based on engaging, applied learning with real-world examples and case studies. Our online training is a highly interactive way to learn at a time & pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.
This course is an internationally accredited credential offering global recognition. CQI and IRCA certify this course (No. 2378) and the Regulatory Affairs Professionals Society recognize this course where members will be eligible for RAPS credits (12).





View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Course Overview
EU MDR Requirements Training
Explain the history, purpose, and structure of the EU MDR, and the key terminology used throughout the regulation
Identify the types of devices covered by the EU MDR and the rules for classifying these devices
Describe the obligations of the economic operators and the PRRC
Describe the General Safety & Performance Requirements and the key features of a risk management system based on ISO 14971
Describe the contents of the Technical Documentation and the requirements for Post Market Surveillance, Vigilance, and Clinical Data
Explain the Unique Device Identifier requirements and the relationship with EUDAMED
This course has been designed for those working in Quality Assurance and/or Regulatory Affairs who want to gain a better understanding of the EU Medical Device Regulation (EU MDR 2017/745), including:
- Quality Assurance Professionals
- Quality Engineers
- Research and Design Engineers
- Internal Auditors
- Quality Managers
- Manufacturing Engineers
- Regulatory professionals
CQI & IRCA certify this course (No. 2378).
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (12).
Upon successful completion, each Learner shall receive a digital Certificate of Achievement within 1 business day.
Before completing this course, each Learner should have the following prior knowledge:
- A working knowledge of ISO 13485, which may be gained by completing Comply Guru’s ISO 13485 Foundation Course
- The relationship between ISO 13485 and the EU MDR 2017/745
- Commonly used quality management terms and definitions within ISO 13485 and ISO 9000
- A working knowledge of risk management principles related to the design of a medical device, through ISO 14971
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based)
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
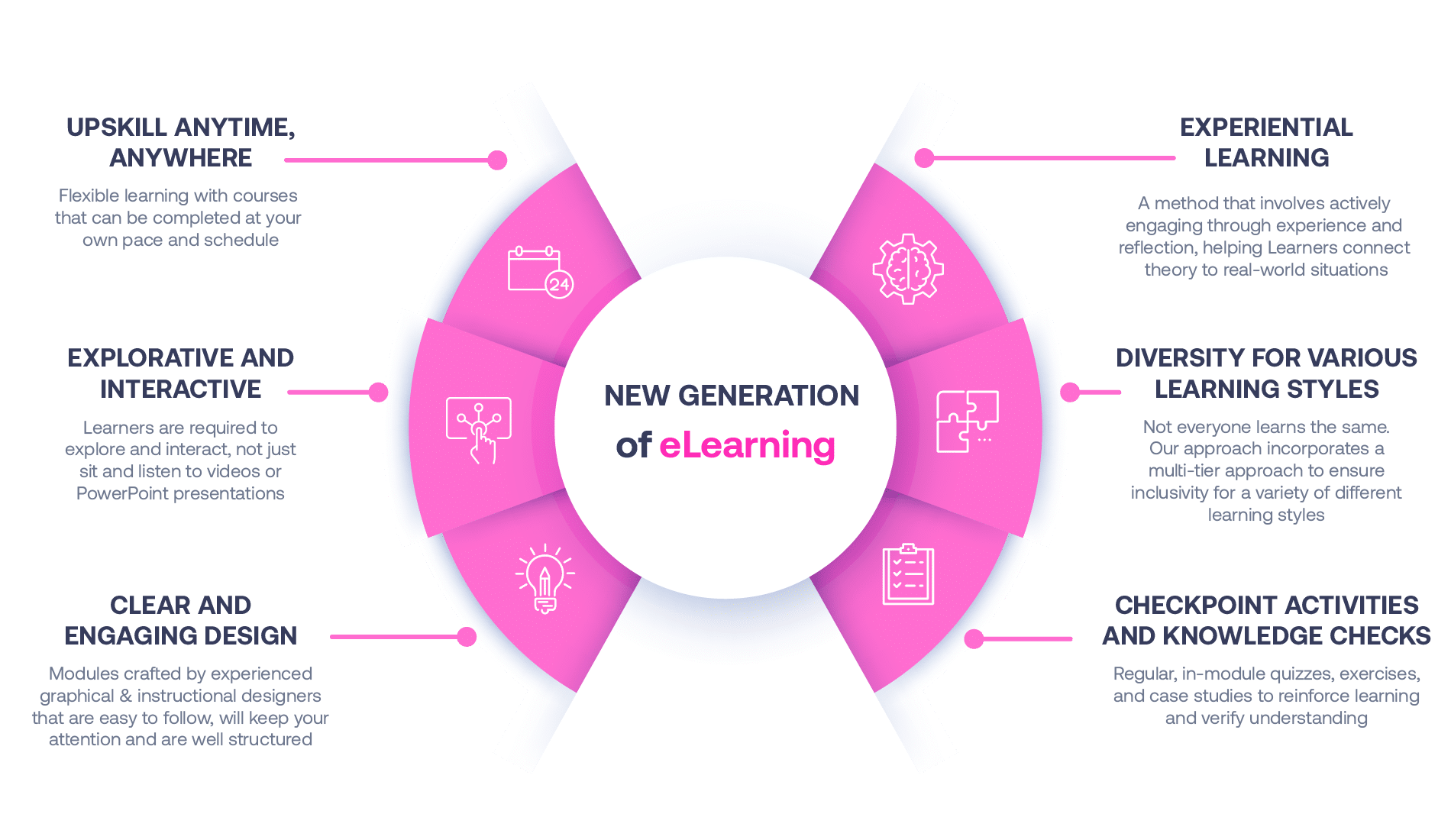
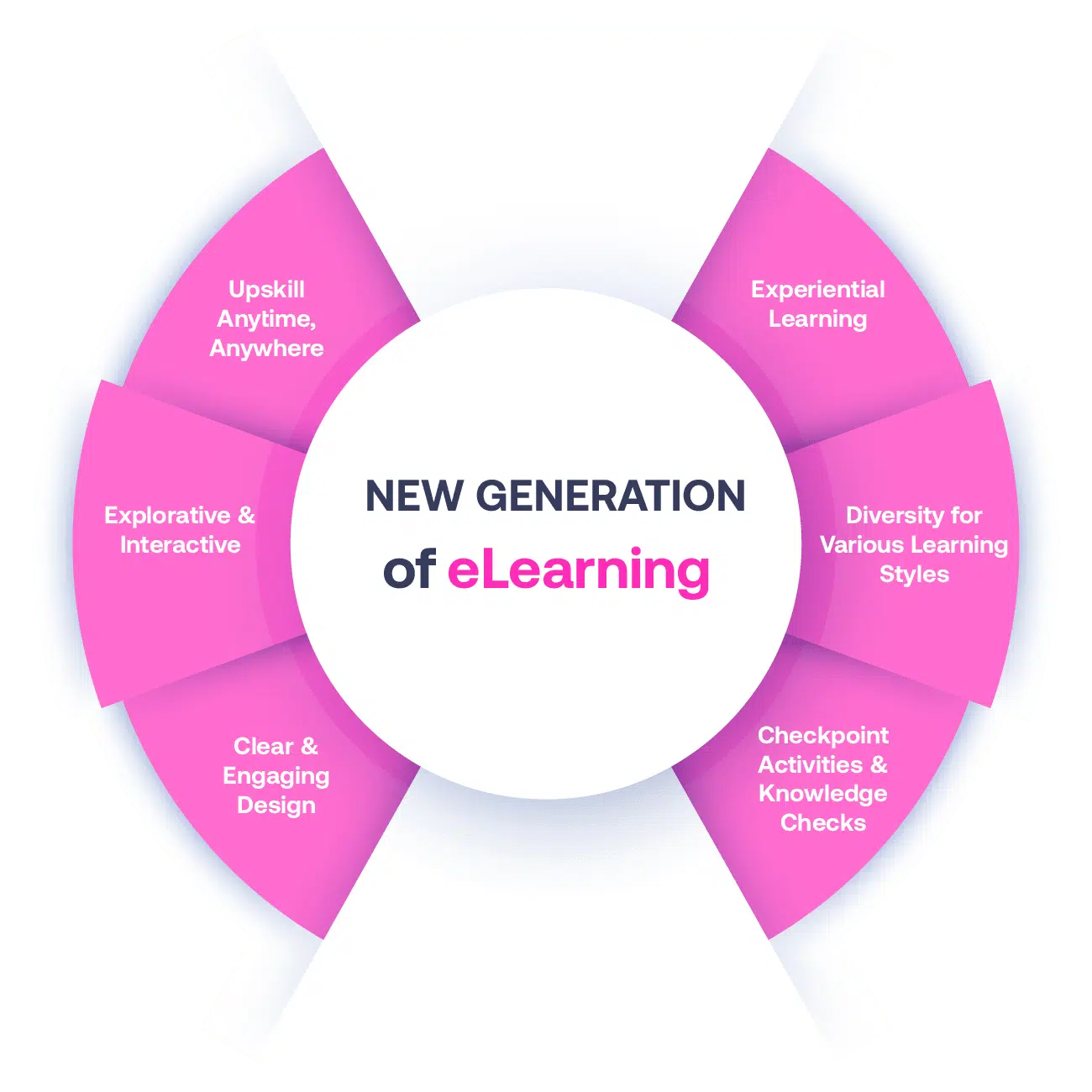
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. Our experienced team of subject matter experts, graphical & instructional designers, and training specialists deploy a multi-layered methodology that offers you a New Generation of eLearning.
Course Structure Explained
eLearning Module Breakdown & Timings
An interactive, engaging eLearning experience that you can complete Anytime, Anywhere.
| Time | Topic |
|---|---|
| 90mins eLearning | Module 1: Introduction to the EU MDR
|
| 95mins eLearning | Module 2: Medical Devices covered by the EU MDR
Case Study! Medical Device Scope Case Study! Scope and Legacy Devices |
| 175mins eLearning | Module 3: Placing a Device on the Market
Case Study! Qualifications of the PRRC Case Study! Change of Authorized Representative Case Study! Declaration of Conformity Case Study! A PRRC under Pressure |
| 45mins | Certification Exam 1 of 3
|
| 160mins | Module 4: Device Classification
Case Study! Device Classification |
| 160mins | Module 5: Routes to Conformity
Case Study! Route to Conformity |
| 150mins | Module 6: GSPR and Risk Management
Case Study! GSPRs 1 – 8 |
| 45mins | Certification Exam 2 of 3
|
| 160mins | Module 7: Clinical Evaluation
Case Study! Excerpt of a CER for Review |
| 150mins | Module 8: Post Market Surveillance and Vigilance
Case Study! Periodic Safety Update Report |
| 145mins | Module 9: Technical Documentation, UDI and EUDAMED
|
| 45mins | Certification Exam 3 of 3
|
Our Experts
Meet The People Behind The Course
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training
Watch and Learn More
About Our Training for Practitioners
Learn about how our IRCA Certified MDR Training for Practitioners is leading the industry for innovation through online learning
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.6
Average Rating
369 global ratings
-
It was an amazing experience. Totally worth the price. The only request would be that, the final assessment could have had lot of case study questions rather than just direct ones. Also, if possible it woudl be nice to get some exmaples of real documents like DoCs, SSCPs etc.
-
The course provides an excellent overview of the MDR and offers additional resources to support a full understanding of the new requirements, as well as guidance for specific scenarios. It will definitely contribute to enhancing my career.
-
My overall training experience with Comply Guru was excellent. The content was clear, well structured, and delivered in a way that made even complex topics easy to understand. The platform was intuitive, the pace was manageable, and the practical examples helped reinforce key concepts.
-
I thought there were good examples and I enjoyed the knowledge checks as they were helpful and practical.
-
The course content and supporting material and the methods used to share the key elements of EU MDR all combined with the perfect blend of learning methods I found made for a very enjoyable and informative course. I remained easily engaged throughout and am a confident practitioner now of EU MDR.
Frequently Asked Questions