Authorized Representative Training
Authorized Representative Training that provides learners with clarity on the role and responsibilities of the Authorized Representative under Article 11 and Article 12 of the Medical Device Regulation (MDR) and In Vitro Diagnostics Regulation (IVDR).
Our training is for anyone that wants to understand the Authorized Representative role under the MDR and IVDR and it can be completed in-person or virtually.




Download Brochure
Have 5 or more that need training?
We offer group discounts and are available for in-house (live or virtual) or tailored training in multiple formats.
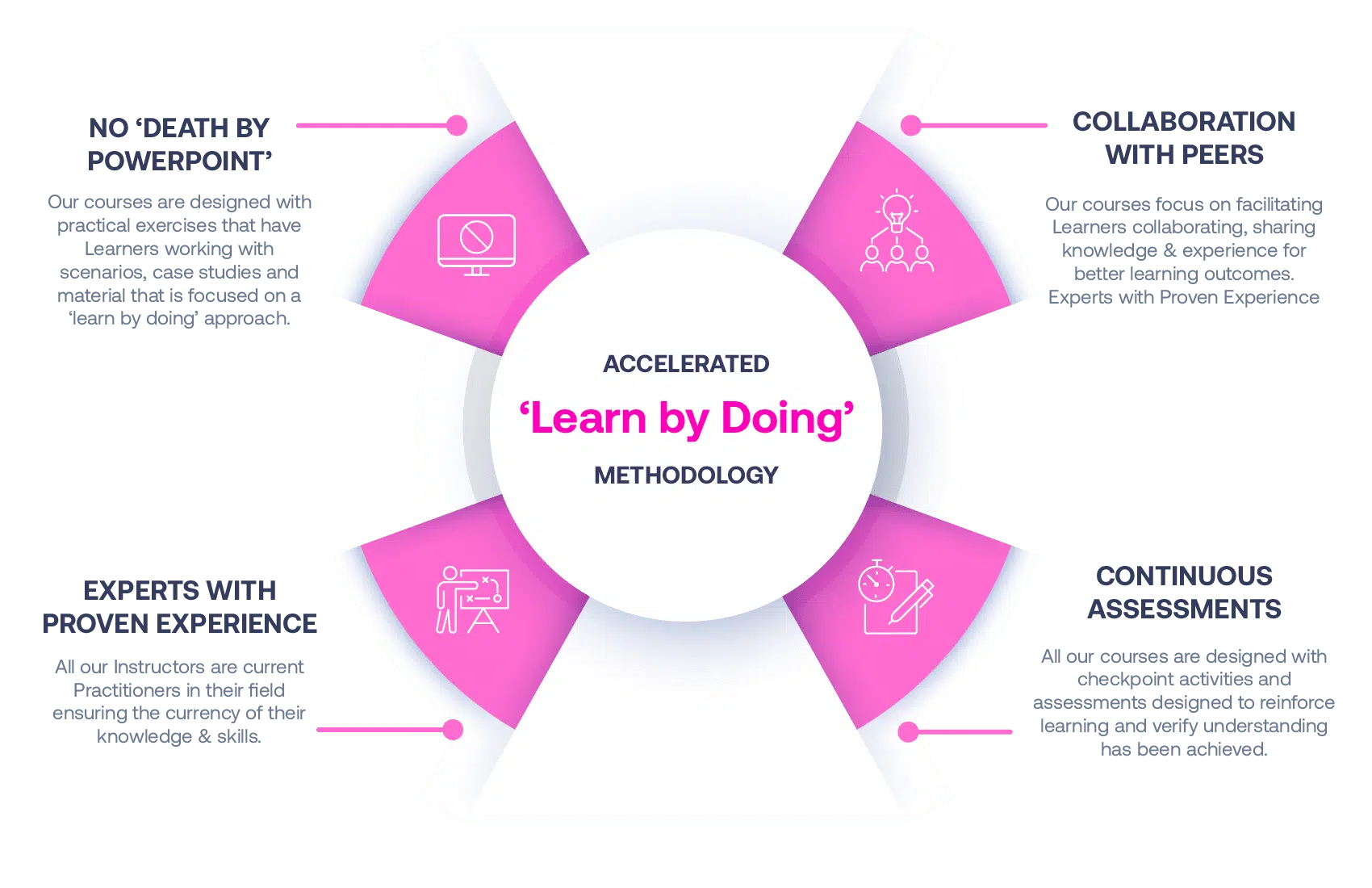
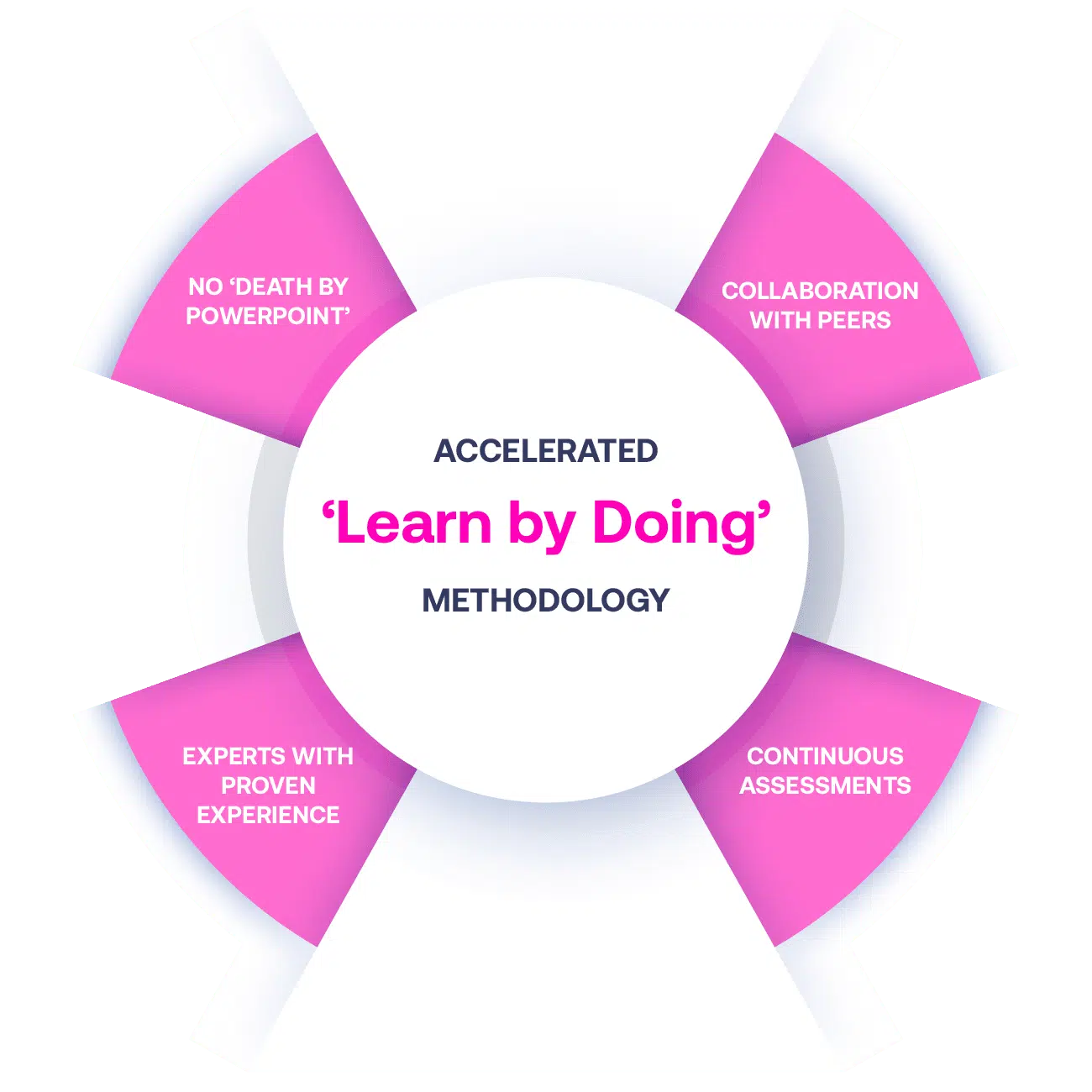
Our Methodology
No More Death By PowerPoint
Our courses are designed with practical exercises that have Learners working with scenarios, case studies and material that is focused on a ‘learn by doing’ approach.
Our Experts
Meet The Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Course Overview
Authorized Representative Training
On completion of this course, successful Learners will have the knowledge needed to:
- Understand the function and the role of the Authorized Representative
- Identify the relationship between the Authorized Representative and the Manufacturers QMS and Documentation (Technical File and Declaration of Conformity)
- Recognize the requirements of Registration Obligations
- Understand the responsibilities of the Authorized Representative in respect of Post Market Surveillance and Vigilance
- Understand the relationship between the Authorized Representative, Competent Authorities, and the Manufacturer
- Understand the Liability of the Authorized Representative
- Understand the process of Changing the Authorized Representative
A summary of learning topics within this course include:
- The Role of the Authorized Representative in the context of Article 11 and Article 12 MDR/IVDR
- Economic Operators of (EU) 2017/745 MDR
- The role of the Authorized Representative with the Competent Authorities
- Responsibilities of the Authorized Representative
- The Authorized Representative, Post Market Surveillance and Vigilance
- Liability for the Authorized Representative
- The Mandate between the Authorized Representative and the Manufacturer
- Obligations of the Authorized Representative in respect of the PRRC
- Task of the PRRC of an Authorized Representative
- EUDAMED Registration Obligations
- Documentation and the Authorized Representative
- Document Retention Periods for the Authorized Representative
- Quality Assurance Professionals
- Quality Engineers
- Regulatory Professionals
- Internal Auditors
- Lead Auditors
- Management Representatives
- Top Management
Successful completion will entitle each Learner to receive a digital Certificate of Completion within 1 business day.
In order to successfully complete this course, each Learner will need to:
- Fully attend the Live Instructor Workshop as 100% attendance is required
- Pass the end of course final assessment.
Prior to attending this course, learners must be informed that they are expected to have the following prior knowledge:
EU MDR or IVDR
- A good understanding of the EU MDR (2017/745) and/or EU IVDR (2017/746) requirements and their application.
ISO 13485
- Must have experience of working with ISO 13485
Medical device management systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
If you are completing this training virtually, the following applies:
For the live workshops during a virtual delivery, we utilize both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Watch and Learn More
About our Approach to Training
Learn about the benefits and key features of Comply Guru’s Instructor led Learning.