EU IVDR Training for Practitioners working with the European In Vitro Diagnostic Regulation (2017/746)
EU IVDR Training that takes you through the new European In Vitro Diagnostic Regulation (2017/746), not just from an industry perspective, but also from a Notified Body perspective. It has been specifically designed for Quality & Regulatory Professionals who are new to the Regulation.
Our online training is a highly interactive way to learn at a time & a pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.
The Regulatory Affairs Professionals Society (RAPS) recognize this course offering global recognition for successful participants and RAPS credits for members.





Download Brochure
View Sample Certificate of Achievement


Have 5 or more that need training?
We offer group discounts and are available for in-house (live or virtual) or tailored training in multiple formats.
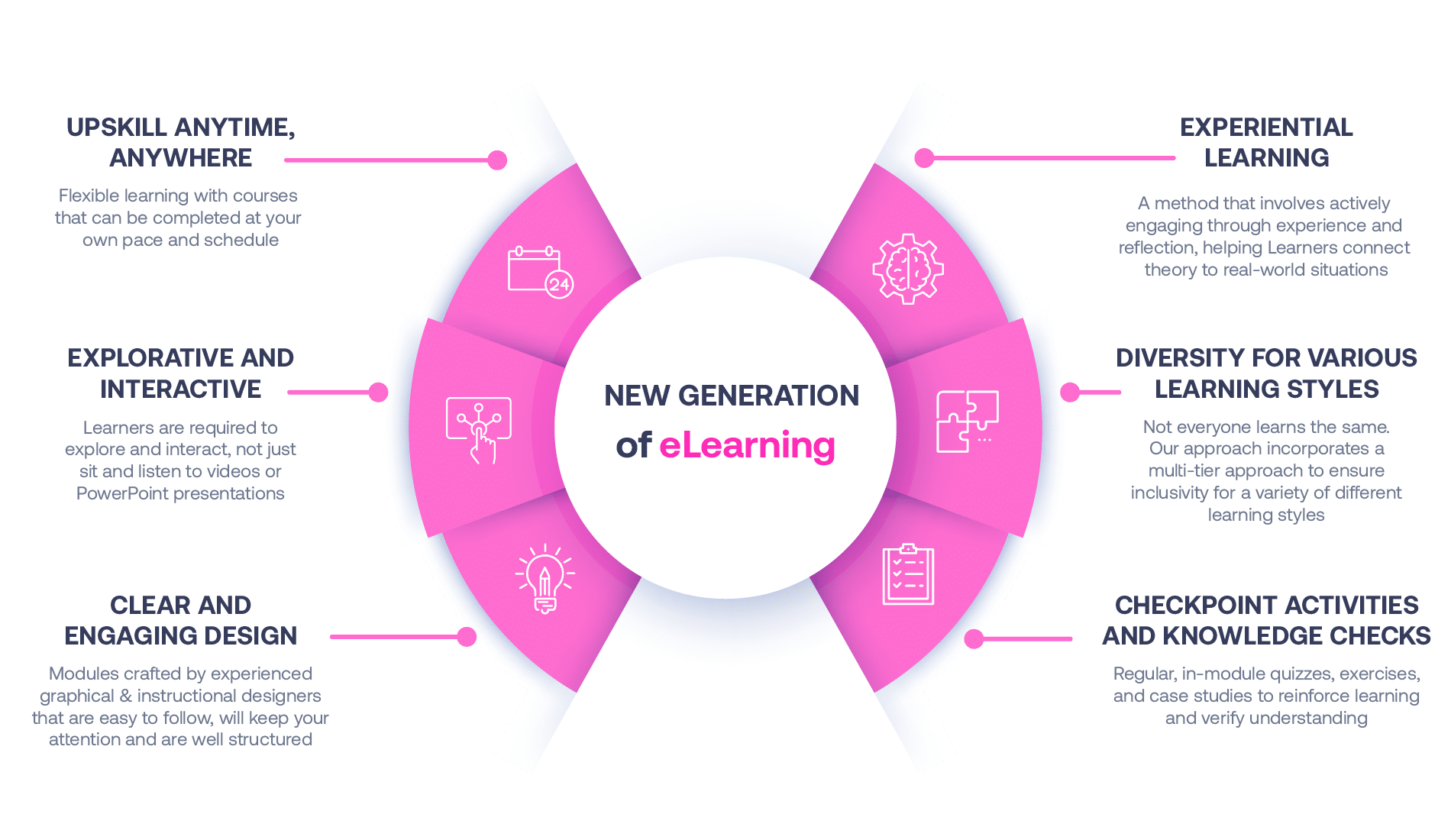
Our Methodology
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Course Structure Explained
eLearning Module Breakdown & Timings
An interactive, engaging eLearning experience that you can complete Anytime, Anywhere.
| Time | Topic |
|---|---|
| 90mins eLearning | Module 1: Introduction to the EU IVDR
|
| 95mins eLearning | Module 2: Medical Devices covered by the EU IVDR
|
| 175mins eLearning | Module 3: Placing a Device on the Market
|
| 45mins | Certification Exam 1 of 3
|
| 160mins | Module 4: Device Classification
|
| 160mins | Module 5: Routes to Conformity
|
| 150mins | Module 6: GSPR and Risk Management
|
| 45mins | Certification Exam 2 of 3
|
| 160mins | Module 7: Clinical Evidence, Performance Evaluation & Performance Studies
|
| 150mins | Module 8: Post Market Surveillance and Vigilance
|
| 145mins | Module 9: Technical Documentation, UDI and EUDAMED
|
| 45mins | Certification Exam 3 of 3
|
Our Experts
Meet The People Behind The Course
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Course Overview
EU IVDR Training for Practitioners
Explain the history, purpose, and structure of the EU IVDR and the key terminology used throughout the regulation
Identify the types of the device covered by the EU IVDR, the rules for classification, and the routes to conformity
Describe the obligations of the economic operators and the PRRC
Describe the General Safety & Performance Requirements
Outline the requirements for Performance evaluation and Performance studies
Explain the Post Market Surveillance and Vigilance reporting requirements
Describe the contents of the Technical Documentation and explain the Unique Device Identifier requirements
This course is aimed at anyone working in the in-vitro diagnostic medical device sector who is responsible for or involved in ensuring compliance to the EU IVDR 2017/746 including but not limited to:
- Regulatory affairs
- Design and development
- Quality Management/Assurance
- Quality Engineers
- Internal Auditors
- Authorized Representatives
- PRRCs
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (12).
Successful completion will entitle each Learner to receive a digital Certificate of Achievement.
Before completing this course, each Learner should have the following prior knowledge:
- Knowledge of the requirements of ISO 13485, which may be gained by completing a CQI and IRCA Certified MD-QMS ISO 13485 Foundation (FD132) training course or equivalent.
- The relationship between ISO 13485 and the EU IVDR 2017 746
- Commonly used quality management terms and definitions within ISO 13485
- A working knowledge of risk management principles related to the design of a medical device, through ISO 14971
In order to successfully complete this course, each Learner will need to:
- Complete all the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based)
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Watch and Learn More
About Our Training for Practitioners
Learn about how our Accredited IVDR Training for Practitioners is leading the industry for innovation through online learning
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.5
Average Rating
71 global ratings
-
This course provided a good understanding of the IVDR and how this can be applied in an organisation.
-
Really thorough. I needed the course for certain aspects so some sections were irrelevant.
-
I feel more equipped to deal with IVDR than before thanks to this course. I may need to review the course again do to the size of the topic.
-
The format of this training perfectly suited my learning style. The knowledge checks pushed me to think critically about the regulation and reinforced my understanding of the IVDR requirements. Highly recommend this course for anyone looking to strengthen their expertise in this area.
-
Overall the course was a great way to gain an understanding of the IVDR.