ISO 13485 Internal Auditor Training for Medical Device Quality Management Systems (MD-QMS)
ISO 13485 Internal Auditor Training is for anyone that wants to be part of the team that conducts internal audits of part of their Quality Management System (QMS) based on ISO 13485.
Our eLearning courses are a highly interactive way to learn at a time & a pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.






View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Course Overview
ISO 13485 Internal Auditor Training
On completion of this course, successful Learners will have the knowledge needed to:
- Explain the purpose of a medical device quality management system, of medical device quality management systems standards, and the business benefits of improved performance given by an effectively implemented medical device quality management system
- Outline the structure and content of ISO 13485 and its relationship with ISO 9001 and IMDRF individual country regulatory documents
- Explain the specific quality management-related requirements of ISO 13485
- With reference to the PDCA cycle, explain the process-based QMS Model for ISO 13485 and the role of an internal auditor in the maintenance and improvement of Quality Management Systems
- Explain the role and responsibilities of an auditor to plan, conduct, report, and follow up an internal audit in accordance with ISO 19011
A summary of learning topics within this course include:
- Purpose, Benefits & Structure of ISO 13485
- Quality Management Principles
- Terms & Definitions
- Documentation Requirements
- Plan-Do-Check-Act (PDCA)
- Process-based Quality Management Systems
- Risk Based Thinking (RBT)
- Management Commitment
- Management System Audits
- Audit Terminology
- Principles of Auditing
- Audit Program Management
- Certification Audit Program
- Audit Life Cycle
- Audit Scope, Objectives & Criteria
- Selection of Audit Location
- Selection of Audit Team
- Auditee, Observer & Guides
- Audit Initiation & Preparation
- The Audit Plan
- Audit Documentation

Exemplar Global certify this course under the Recognized Training Provider scheme. Learners will receive a certificate upon completion with their logo on their digital Certificate of Completion.
Before completing this course, each Learner should have the following prior knowledge:
- It is recommended that anyone wishing to attend this course has some work-based experience in Quality Management (based on ISO 13485)
- The Plan, Do Check, Act (PDCA) Cycle
- The core elements of a management system and the interrelationship between top management responsibility, policy, objectives, planning, implementation, measurement, review, and continual improvement.
Fluency in written and spoken English
- For participants whose first language is not English, we recommend a minimum English language competency of IELTS 5.5 (or equivalent) for successful completion of the program. This is not assessed by Comply Guru in advance & each participant must self-assess their competency.
Copy of ISO 13485 (International Standard)
- It is recommended that each Learner should have a copy of ISO 13485:2016 (International Standard) to reference while completing this course. This is not provided by Comply Guru and without it, this course will be challenging to complete successfully.
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based)
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
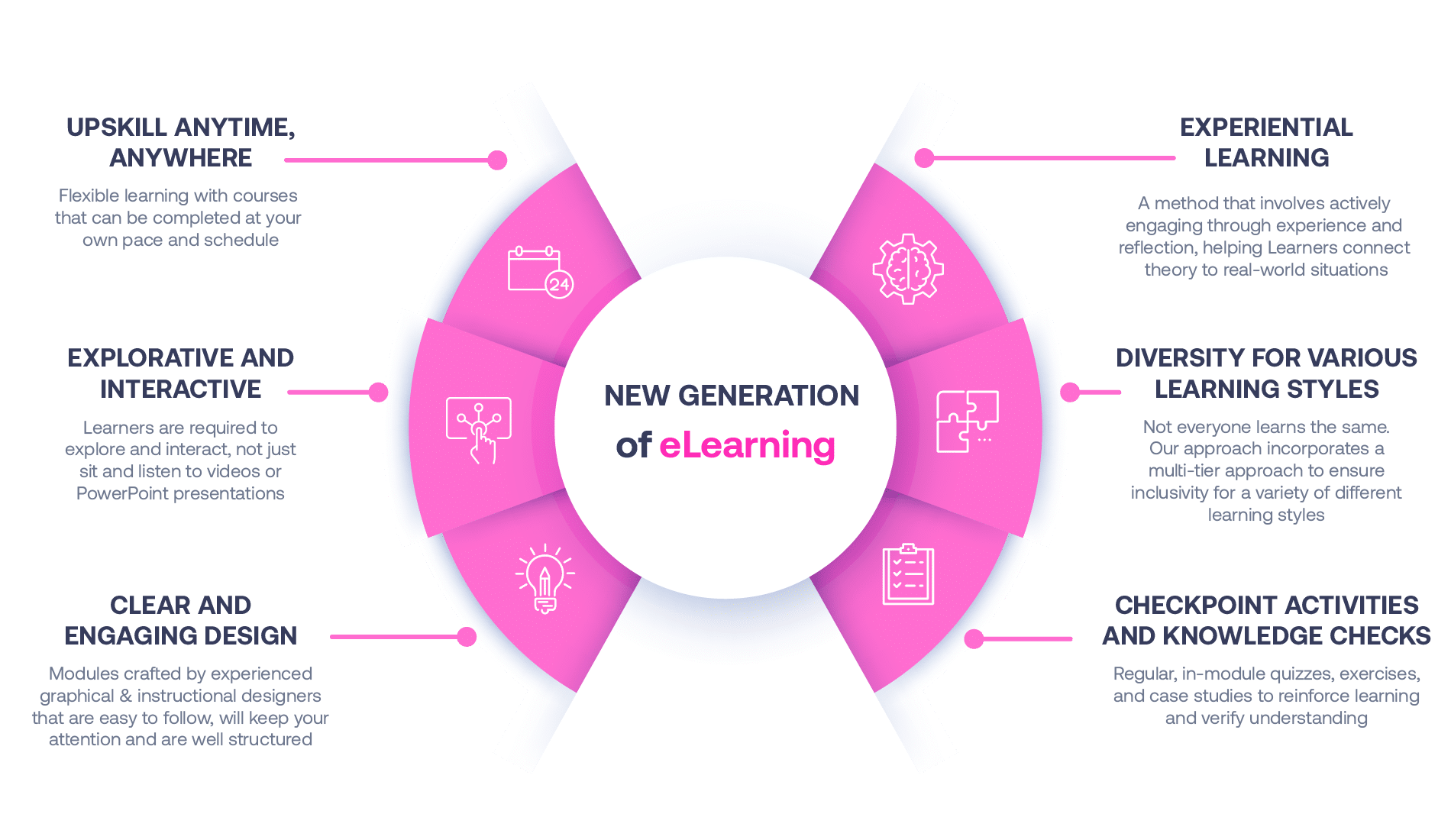
Our Methodology
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Course Structure Explained
eLearning Module Breakdown & Timings
An interactive, engaging eLearning experience that you can complete Anytime, Anywhere.
| Time | Topic |
|---|---|
| 70mins eLearning | Module 1 – Overview of ISO 13485
Case Study! Risk Management |
| 110mins eLearning | Module 2 – Establishing a Quality Management System
Case Study! Software Validation Case Study! Record Control |
| 65mins eLearning | Module 4 – Resource Management
Case Study! Training and Document Updates Case Study! Work Environment and Contamination Control |
| 95mins eLearning | Module 5 – Product Realization, Part 1
Case Study! Customer Related Processes |
| 70mins eLearning | Module 6 – Product Realization, Part 2
Case Study! Purchasing Controls |
| 70mins eLearning | Module 7 – Measurement, Analysis, and Improvement
Case Study! Complaint Handling Case Study! Internal Audits |
| 45mins | Requirements Exam
|
| 120mins eLearning | Module 8: Introduction to Auditing
|
| 40mins eLearning | Module 9: Audit Initiation and Preparation
|
| 80mins eLearning | Module 10: Conducting Audit Activities
|
| 25mins eLearning | Module 11: Audit Report, Close and Follow Up
|
| 40mins | Internal Auditor Exam
|
Our Experts
Meet The People Behind The Course
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About Our ISO 13485 Internal Auditor Training
Learn about how our Accredited ISO 13485 Internal Auditor Training is leading the industry for innovation & learning effectiveness
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.4
Average Rating
85 global ratings
-
Very clear and concise instruction, easy to follow, highly recommend.
-
Great learning tool
-
Excellent course. I would definitely recommend to others looking to refresh their knowledge on ISO and Internal auditing.
-
I was able to increase my understanding of Internal Auditing and ISO 13485 through completing this course. I found being able to complete the course content online, at my own pace especially beneficial as I could fit the time needed to complete the course into my already busy daily schedule.
-
Training was satisfactory
Frequently Asked Questions