Post Market Surveillance (PMS) under EU MDR 2017/745 for Practitioners
Post Market Surveillance (PMS) under the EU Medical Device Regulation (MDR) Training for Practitioners is for those looking to master the PMS requirements under the EU MDR 2017/745
This interactive course is designed to equip quality and regulatory professionals, medical device manufacturers, and post-market teams with the knowledge and tools to meet these rigorous requirements. From understanding the legal framework to developing a compliant PMS Plan, this course covers every essential aspect of PMS under EU MDR.
With real-world examples, practical activities, and scenario-based learning, you’ll gain the skills to confidently implement PMS processes, generate required reports, and stay prepared for audits and inspections. Whether you’re new to PMS or looking to enhance your expertise, this course provides the insights you need to ensure compliance and protect patient safety.
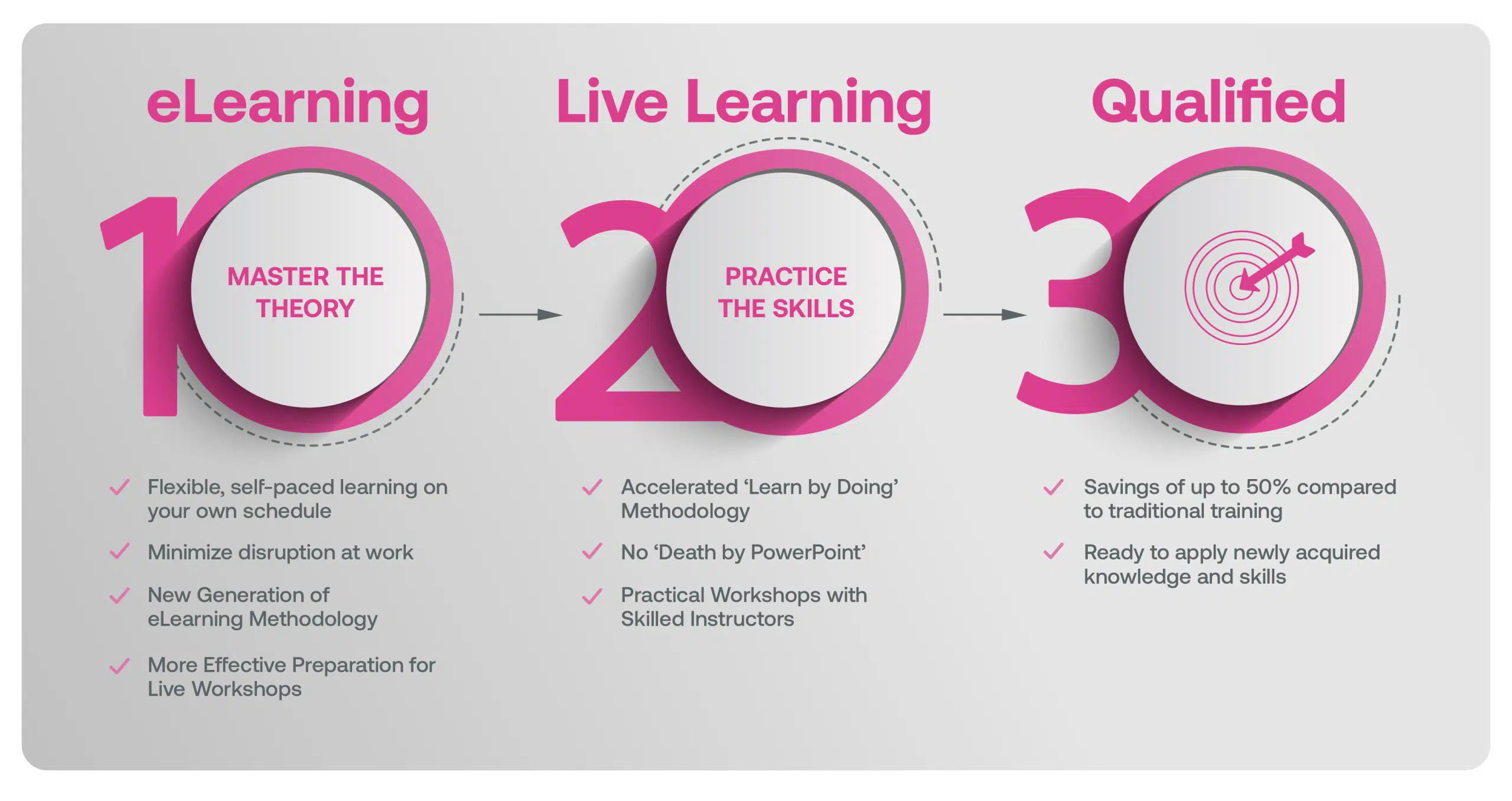
Our blended learning course is a hybrid approach where you first complete eLearning modules on the underlying theory that better prepares you in advance of attending Live Classes with an Instructor that focus on practical application in a real life context.
This training course recognized by the Regulatory Affairs Professionals Society (RAPS) offering global recognition for successful learners. RAPS members will also be eligible to receive 12 credits by completing this course.





View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain a Recognized Qualification
Advance your knowledge and upskill with one of our Instructors.

Register 3 & Get 1 Free

Course Overview
Post Market Surveillance (PMS) Practitioner
On completion of this course, successful Learners will have the practical knowledge and be able to apply the techniques needed to:
- Define Post-Market Surveillance (PMS) and its significance under EU MDR 2017/745.
- Identify PMS requirements for medical device manufacturers.
- Develop and implement a compliant PMS Plan.
- Evaluate and report PMS findings through PSURs and PMSRs.
- Align PMS activities with other MDR processes, including vigilance and risk management.
- Quality Professionals
- Quality Engineers
- Regulatory Professionals
- Internal Auditors
- Lead Auditors
- Management Representatives
- Top Management
EU MDR 2017/745
- Familiarity with the structure and key provisions of the EU Medical Device Regulation 2017/745.
- Basic knowledge of medical device classification and conformity assessment procedures under EU MDR.
ISO 13485
- Must have experience of working with ISO 13485:2016 or knowledge of ISO 13485:2016
Medical device management systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
- The process approach used in quality management.
ISO 14971
- Basic understanding of ISO 14971
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (12).
Successful completion will entitle each Learner to receive a digital Certificate of Completion within 1 business day.
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based) by the required deadline set in advance of the given workshop dates you are registered for (applies to blended format only)
- Fully attend the Instructor Workshops as 100% attendance is required
- Obtain 70% or higher in the graded assessments during the Instructor Workshops
If you are completing this training virtually, the following applies:
For the live workshops during a virtual delivery, we utilize both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
Blended Learning is Better Learning
A two-step methodology with eLearning modules that help you master the theory better preparing you for practical workshops that embed the skills.
Course Structure Explained
Detailed Breakdown & Agenda
Learners first complete interactive eLearning modules to grasp the underlying theory, then attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| 40mins | Module 1: Introduction to the EU MDR 2017/745 and PMS
|
| 90mins | Module 2: Legal and Regulatory Framework for PMS
Case Study Analyze a scenario where a device’s PMS obligation were not met and the resulting consequences. |
| 55mins | Module 3: Developing a PMS Plan
Case Study Review of a PMS Plan |
| 80mins | Module 4: Conducting PMS Activities
Case Study Identifying gaps in PMS Data Collection |
| 55mins | Module 5: PMS Reporting Requirements
Case Study Identify the correct reporting requirements for different device classes |
| Time | Topic |
|---|---|
| 09:00am - 17:00pm | Following completion of the eLearning modules where Learners master the theory, an experienced Practitioner will lead practical workshops diving deeper into PMS and practical application covering:
|
Our Experts
Meet The Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About our Post Market Surveillance Training
Learn about the benefits and key features of Comply Guru’s Blended Learning.
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.8
Average Rating
6 global ratings
-
The course really helped me get an overall understanding of the PMS under MDR that I can apply in my current role.
-
Excellent course content with the participants interaction supporting each other understand the requirements.
-
I thoroughly enjoyed the training course. The blended learning approach with no PowerPoint slides was a refreshing approach. The trainer was very knowledgeable on the topic and very personable. her energy in delivering the course kept us all engaged and elicited contributions from all participants.
-
An excellent interactive course with a very knowledgeable instructor.
-
very useful and informative