EU MDR Internal Auditor Training for the European Medical Device Regulation (2017/745)
EU MDR Internal Auditor Training for existing ISO 13485 Internal or Lead Auditors seeking the knowledge & skills to perform internal audits of medical device quality management systems (MD-QMS) against the European Union’s Medical Device Regulation (EU MDR 2017/745) in accordance with ISO 19011.
Our live learning methodology focuses on practical ‘learn by doing’ workshops set in a real life context covering:
- Refresh understanding of Key MDR Requirements not covered under ISO 13485
- How to review documents in advance of an MDR Internal Audit
- Developing an MDR-focused audit plan and audit checklist
- Audit situations and identification of MDR related nonconformities
- Writing clear audit findings linked to MDR clauses
- How to manage corrective actions, assess effectiveness, and consider regulatory implications
- Develop audit findings and a short report based on an MDR-based findings





Download Brochure
View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Course Overview
EU MDR Internal Auditor Training
Refresh understanding of the key MDR requirements including Device Classification, Routes to Conformity, GSPR and Risk Management, CER, PMS and Vigilance
Explain the role and responsibilities of an internal auditor to plan, conduct, and report nonconformities for a quality management system audit in accordance with ISO 19011 that uses the EU MDR 2017/745 requirements as the audit criteria.
Plan for and conduct an internal audit of a medical device quality management system to establish compliance with the EU MDR 2017/745 in accordance with ISO 19011 as applicable and report on any nonconformities.
Upon successful completion, each Learner shall receive a digital Certificate of Achievement.
Prior to attending this course, learners are expected to have the following prior knowledge:
EU MDR
- A good understanding of the EU MDR (2017/745) requirements and their application is required for this course. This may be gained by successfully completing CQI and IRCA Certified MD-QMS Comprehensive EU-MDR 2017/745 Practitioner (PT219) course.
Please note: if no recognized certificate is provided, additional information & a pre-course test may be required to verify knowledge of the requirements in order to be accepted onto the course.
ISO 13485
- Must have successfully completed a recognized MD-QMS ISO 13485 Internal or Lead Auditor course or equivalent.
Medical device management systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
- The process approach used in quality management.
- A working knowledge of risk-management principles related to the design of a medical device, through ISO 14971.
To successfully complete this course, each Learner will need to:
- Fully attend the Instructor Workshops as 100% attendance is required.
- Obtain 70% or higher in the graded assessments during the Instructor Workshops
For the live workshops during a virtual delivery, we utilise both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
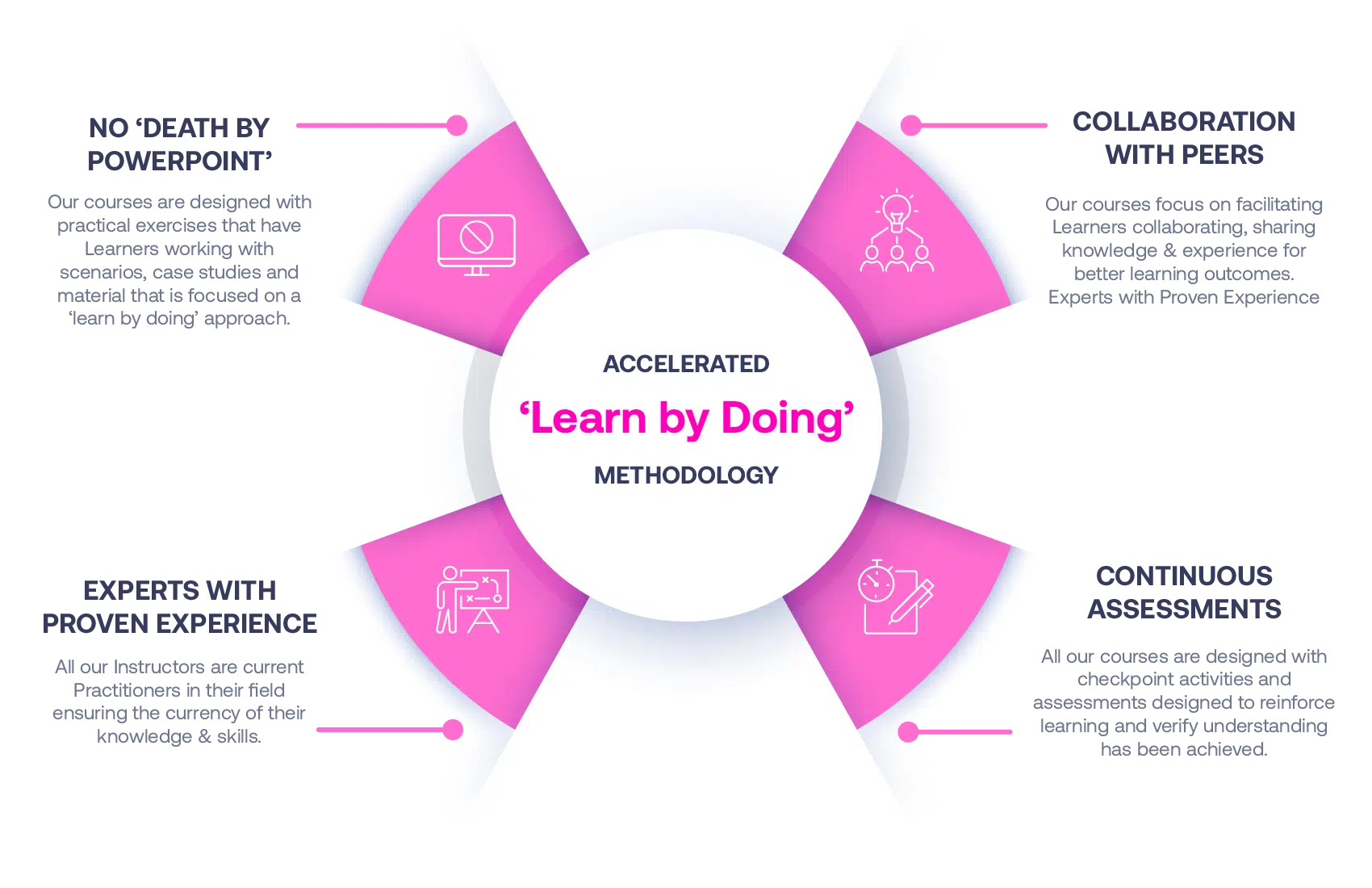
Our Methodology
No More Death By PowerPoint
Our courses are designed with practical exercises that have Learners working with scenarios, case studies and material that is focused on a ‘learn by doing’ approach.
Course Structure Explained
Detailed Breakdown & Agenda
Attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| 9:00am | Course Introduction
|
| 10:00am | Break |
| 10:15am | Device Classification & Routes to Conformity |
| 11:15am | Break |
| 11:30am | GSPR and Risk Management |
| 12:30pm | Lunch |
| 1:15pm | Clinical Evaluation, Post Market Surveillance and Vigilance |
| 2:15pm | Break |
| 2:30pm | Audit Preparation – Document Review
|
| 3:30pm | Break |
| 3:45pm | Day 1 Wrap Up
|
| 4:00pm | End of Day 1 |
| Time | Topic |
|---|---|
| 9:00am | Audit Preparation – Plan & Checklist
|
| 10:00am | Break |
| 10:15am | MDR Based Audit Scenarios
|
| 11:15pm | Break |
| 12:15pm | Audit Findings & Report Writing
|
| 1:15pm | Lunch |
| 2:00pm | Follow-up Activities
|
| 3:00pm | Break |
| 3:15pm | Scenario Based Exercise – Audit Findings & Report
|
| 4:15pm | Break |
| 4:30pm |
|
| 5:00pm | End of Course |
Our Experts
Meet the Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About our EU MDR Internal Auditor Training
Learn about the benefits and key features of Comply Guru’s Instructor led Learning.
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.3
Average Rating
22 global ratings
-
Good one, Really helpful when you start your adventure with MDR....
-
Great training experience. Clear expectations from the start and the training instructor was very knowledgeable.
-
Situation case super nice
-
This approach is the best for my learning style. I appreciate the hands on application of this extensive and at times overwhelming regulation.
-
This training provided a lot of good information and guidance on interpretation of EU MDR in the scope of internal auditing.
Frequently Asked Questions