FDA Regulatory Requirements for Medical Devices (United States)
This course provides an overview of the United States FDA Regulatory Requirements for Medical Devices.
Whether you’re navigating premarket submissions, managing labelling and registration, or preparing for post-market obligations, this training equips you with the knowledge you need to ensure compliance.
You’ll explore FDA device classification, key regulatory pathways like 510(k), De Novo, and PMA, and gain clarity on labelling rules, Unique Device Identification (UDI), registration and listing, and adverse event reporting. The course also touches on current FDA trends, digital health considerations, and the FDA’s role in Global Harmonization efforts.
Our eLearning is a highly interactive way to learn at a time & a pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.





Download Brochure
Have 5 or more that need training?
We offer group discounts and are available for in-house (live or virtual) or tailored training in multiple formats.
Course Overview
U.S FDA Regulatory Requirements for Medical Devices
At the end of this short course, successful learners will be able to:
- Understand the FDA’s Regulatory Framework for Medical Devices, including Device Classification and Applicable Market Authorization Pathways
- Identify the requirements for premarket submissions such as 510(k), De Novo, PMA, and related clinical investigation processes
- Apply FDA rules for labelling, Unique Device Identification (UDI), and Establishment Registration and Device Listing
- Recognize Post-Market Regulatory Obligations, including adverse event reporting, recalls, and surveillance requirements
- Navigate current FDA initiatives, digital health considerations, and the agency’s role in Global Regulatory Alignment
This course is for individuals or organizations who want to understand the United States FDA Regulatory Requirements for Medical Devices
Successful completion of the course examination will entitle each Learner to receive a digital Certificate of Completion.
In order to successfully complete this course, each Learner will need to:
- Complete all eLearning modules and obtain 70% or higher in the final assessment (MCQ-based)
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
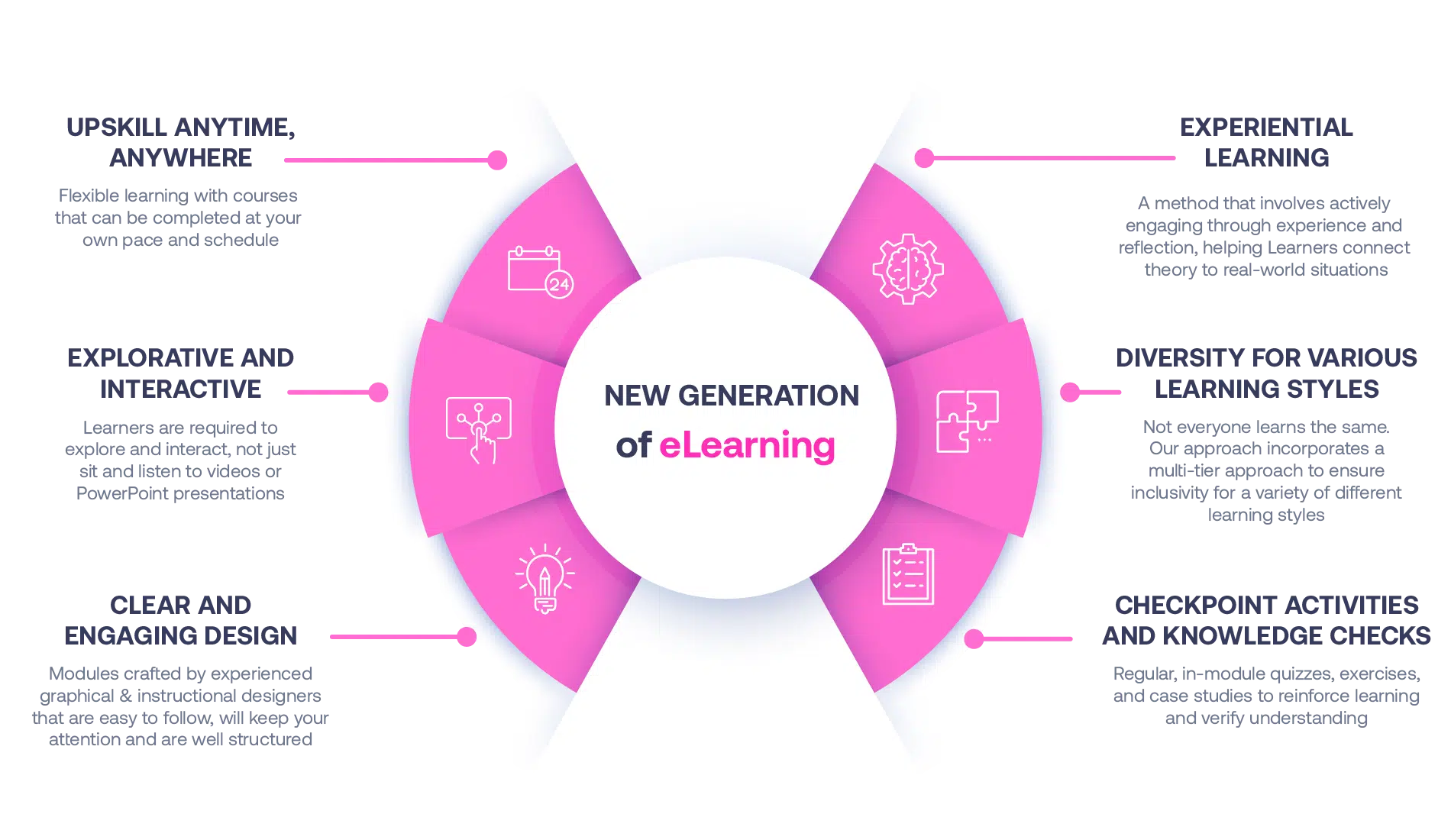
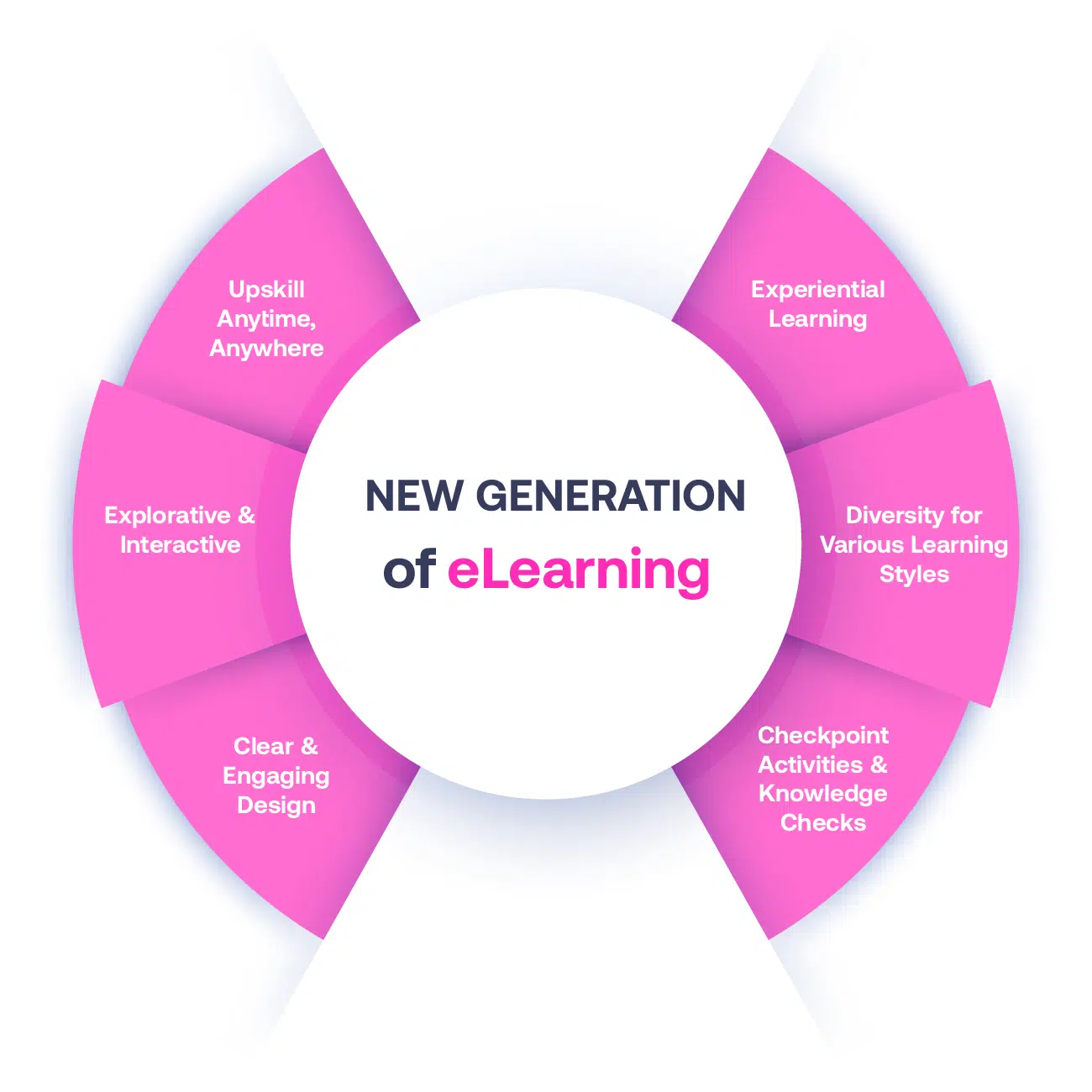
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Our Experts
Meet The People Behind The Course
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About our eLearning
Learn about how our eLearning is leading the industry for innovation through online learning
Frequently Asked Questions