ISO 13485 Lead Auditor Training for Medical Device Quality Management Systems (MD-QMS)
ISO 13485 Lead Auditor Training is for anyone that wants to gain the knowledge and skills required to conduct full system first, second and third-party audits based on ISO 13485, in accordance with ISO 19011 and ISO 17021.
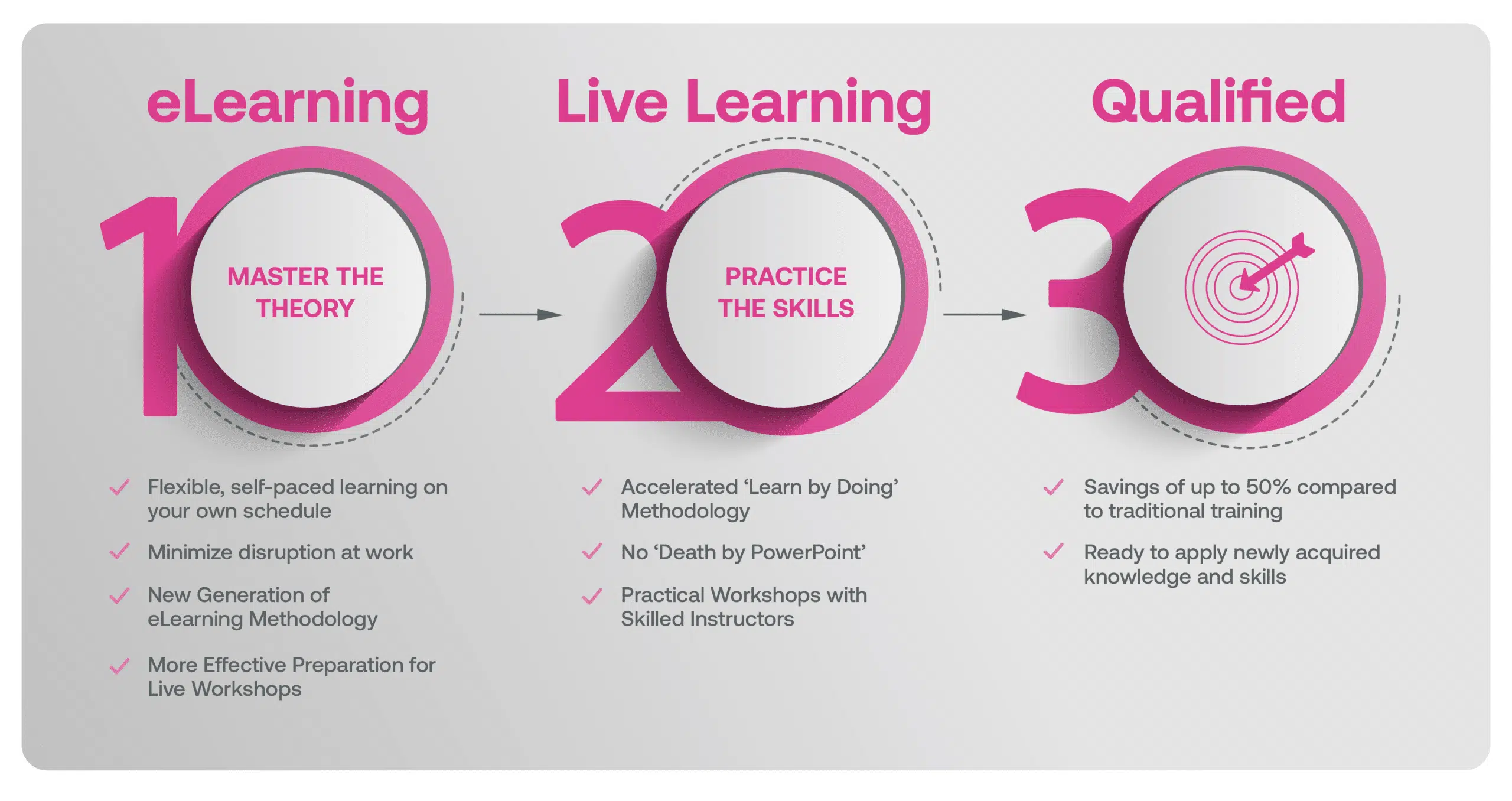
Our blended learning course is a hybrid approach where learners first complete eLearning modules on the underlying theory that better prepares learners in advance of attending Live Classes with an experienced Instructor. Our live workshops are based on practical training methods with real-world examples, case studies, facilitated exercises and group discussions.
CQI and IRCA certify this course (No. 2729) offering global recognition for successful participants.





View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain an Globally Recognized Lead Auditor Qualification
Advance your knowledge and enjoy more flexibility & learning effectiveness with Comply Guru.

Register 3 + Get 1 Free

Course Overview
ISO 13485 Lead Auditor Training
Explain the purpose of a medical device quality management system (MD-QMS), interaction
with appropriate medical device regulatory authority requirements, quality management systems standards, third-party certification, and the business benefits of the quality management system.
Explain the role and responsibilities of an auditor to plan, conduct, report, and follow-up a quality management system audit in accordance with ISO 19011 and ISO/IEC 17021
Plan, conduct, report, and follow-up an audit of a medical device quality management system
to establish conformity (or otherwise) with ISO 13485 and applicable medical device regulatory requirement documents in accordance with ISO 19011 and ISO/IEC 17021
CQI & IRCA certify this course (No. 2729). Upon successful completion, each Learner shall receive a digital Certificate of Achievement.
Before completing this course, each Learner should have the following prior knowledge:
Work Experience
- It is recommended that anyone wishing to attend this course has some work-based experience in Quality Management (based on ISO 13485) or Internal Auditing in advance of registering for this training course
Medical Device Quality Management Systems (MD-QMS)
- The Plan, Do, Check, Act (PDCA) cycle.
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
- Commonly used quality management terms and definitions within ISO 13485 and ISO 9000.
- The process approach used in MD-QMS.
- A working knowledge of medical device regulatory processes applicable to countries the course is designed to cover, including device regulations, regulatory auditing standards and their relationship with ISO 13485.
- A working knowledge of risk-management principles related to the design of a medical device, for example ISO 14971.
ISO 13485
- Knowledge of the requirements of ISO 13485, which may be gained by completing a CQI and IRCA Certified MD-QMS ISO 13485 Foundation (Requirements) course or equivalent.
- Knowledge of the requirements is a key pre-requisite in attending this course as without a good understanding, the course & final examination may be very challenging.
Fluency in Written and Spoken English
- For participants whose first language is not English, we recommend a minimum English language competency of IELTS 5.5 (or equivalent) for successful completion of the program. This is not assessed by Comply Guru in advance & each participant must self-assess their competency.
Printed Copy of ISO 13485:2016
- Each participant should bring a printed clean copy of the standard with them to the course. This is not provided by Comply Guru.
Important Note: The Learners understanding of the prior knowledge requirements will be tested as part of the course continuous assessment & final examination.
Note: The Learner’s understanding of the prior knowledge requirements will be tested as part of the course’s continuous assessment & final examination.
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based) by the required deadline set in advance of the given workshop dates you are registered for (applies to blended format only)
- Fully attend the Live Instructor Workshops as 100% attendance is required
- Obtain 70% or higher in the graded assessments during the Live Instructor Workshops
- Complete a 2hr Final Examination* that is remotely invigilated (Live Proctored). Each Learner must obtain 70% or higher in the Final Examination which is completed securely online (under strict examination conditions).
*Note: the final examination is closed book and will assess each Learner on the full course program – including knowledge of the ISO 13485 Requirements.
For the live workshops during a virtual delivery, we utilise both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
Blended Learning is Better Learning
A two-step methodology with eLearning modules that help you master the theory better preparing you for practical workshops that embed the skills.
Course Structure Explained
Detailed Breakdown & Agenda
Learners first complete interactive eLearning modules to grasp the underlying theory, then attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| Module 1 | Module 1: Overview of ISO 13485
Case Study! Documentation Requirements Case Study! Process Based QMS Case Study! Risk Based Thinking |
| Module 2 | Module 2: Introduction to Auditing
Case Study! Principles of Auditing Case Study! Determining Audit Duration |
| Module 3 | Module 3: Planning the Audit
Case Study! Audit Objectives, Scope and Criteria Case Study! Audit Behaviours x 4 |
| Module 4 | Module 4: Audit Initiation and Preparation
|
| Module 5 | Module 5: Conducting the Audit
Case Study! Communication during an Audit Case Study! Auditee Reactions Case Study! How the Auditor collects and verifies information |
| Module 6 | Module 6: Generating Audit Findings and Closing Meeting
Case Study! Generating Audit Findings Case Study! Non-Conformity Grading |
| Module 7 | Module 7: Audit Reporting, Completion and Follow Up
|
| Time | Topic |
|---|---|
| Workshop 1 | Planning the Audit – Medical Device Context
|
| Workshop 2 | Audit Planning – Regulatory Focus
|
| Workshop 3 | Developing the Audit Plan & Checklist (Part 1)
|
| Day 3 Wrap – up |
|
| Time | Topic |
|---|---|
| Workshop 4 | Developing the Audit Plan & Checklist (Part 2)
|
| Workshop 5 | Audit Checklist Development
|
| Day 4 Wrap – up |
|
| Time | Topic |
|---|---|
| Workshop 6 | Conducting the Audit
|
| Workshop 7 | Simulated Audit – Medical Device Case Study
|
| Workshop 8 | Simulated Audit (Continued)
|
| Day 5 Wrap – up |
|
| Time | Topic |
|---|---|
| Workshop 9 | Simulated Audit (Continued)
|
| Workshop 10 | Generating Audit Findings – Medical Device Examples
|
| Workshop 11 | Audit Conclusion & Closing Meeting
|
| Day 6 Wrap – up |
|
| Time | Topic |
|---|---|
| Workshop 12 | Audit Report and Follow-up Activities
|
| Workshop 13 | Audit Report and Follow-up Activities
|
| Course Close |
|
| Time | Topic |
|---|---|
| Per Schedule | CQI & IRCA Certification Exam (via eAssessment) |
Our Experts
Meet The Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About Our Lead Auditor Training
Learn about how our IRCA Approved Training is leading the industry for innovation & learning effectiveness
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.7
Average Rating
704 global ratings
-
Really good and intensive course. Develops a good understanding of ISO 13485 as well as the how to audit it.
-
My experience with the lead auditor training was both very informative and enjoyable.
-
In person training was excellent. The pace and content was adjusted to our needs. Discussion was engaging and inclusive. I enjoyed the training experience.
-
Very good Course Michelle is very knowlegable and an excellent instructor
-
Michelle was great and very well prepared, all scenarios and topics were evaluated. Deep introduction to the standard was completed which improved the standard requirements. Extensive training but totally worth it. Thank you.