Introduction to CAPA in Medical Devices Training
This eLearning course provides a comprehensive overview of CAPA requirements, common root cause investigation tools, and the CAPA process in Medical Devices. It can be completed Anytime, Anywhere.
Our eLearning is a highly interactive way to learn at a time & pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.
This is the first CAPA training course recognized by the Regulatory Affairs Professionals Society (RAPS) offering global recognition for successful learners. RAPS members will also be eligible to receive 6 credits by completing this course.





Download Brochure
View Sample Certificate of Completion


Have 5 or more that need training?
We offer group discounts and are available for in-house (live or virtual) or tailored training in multiple formats.
Our Methodology
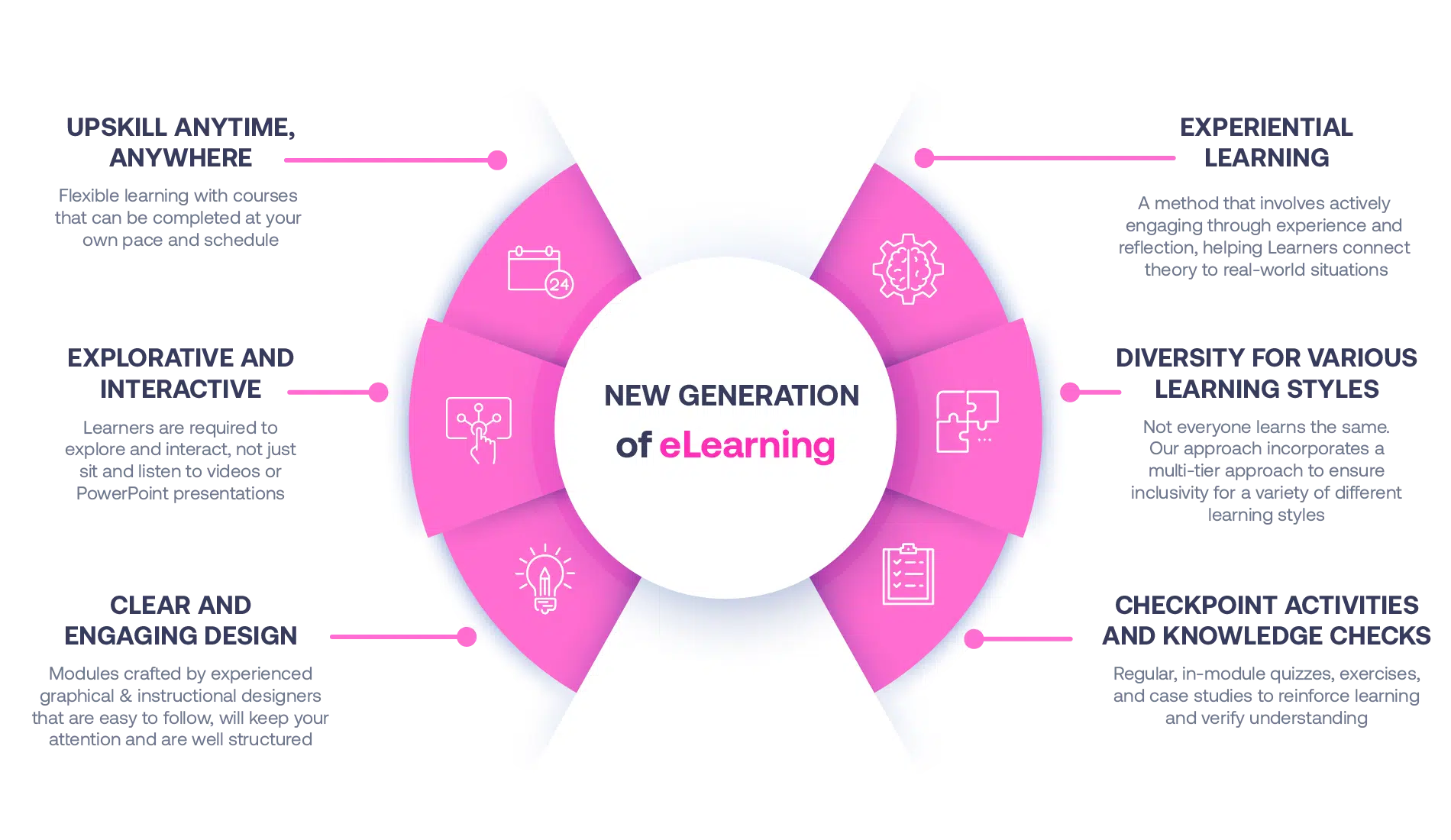
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Course Structure Explained
Detailed Breakdown & Agenda
Our eLearning is a highly interactive way to learn at a time & pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.
| Time | Topic |
|---|---|
| Module 1 | Introduction to CAPA
Case Study A medical device failure and the need for CAPA |
| Module 2 | Corrective Action – Fundamentals
Case Study Conduct a root cause analysis using provided data |
| Module 3 | Preventive Action – Fundamentals
Case Study Proactive Risk Mitigation |
| Module 4 | Integrating CAPA with the QMS
Case Study Identifying gaps in a CAPA process |
| Module 5 | CAPA Process Verification and Monitoring
Case Study Auditing CAPA effectiveness |
Our Experts
Meet The People Behind The Course
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Course Overview
Introduction to CAPA
At the end of this course, Learners will have the knowledge to:
- Understand & implement the Seven CAPA Steps approach.
- Understand & meet FDA and European expectations when completing CAPAs.
- Identify between Correction and Corrective Action.
- Identify between Corrective Action and Preventive Action.
- Understand Corrective Actions that address the Root Causes of failure.
- Understand documentation needed for SMART Effectiveness Checks.
This course would be targeted at those within the MedTech Industry who are involved in any part of a CAPA system, including:
- Operators
- Technical Staff
- Managers
- Quality/Regulatory Affairs Personnel
- Engineering or Support Functions
Before completing this course, each Learner should have the following knowledge and/or experience:
ISO 13485
- Must have experience of working with ISO 13485:2016 or knowledge of ISO 13485:2016
Medical Device Quality Management Systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices
- The process approach used in quality management
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (6).
Successful completion will entitle each Learner to receive a digital Certificate of Completion within 1 business day.
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessment (MCQ-based)
If you are completing this training virtually, the following applies:
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Watch and Learn More
About our CAPA Training
Learn about how our CAPA Training is leading the industry for innovation through online learning
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
5
Average Rating
3 global ratings
-
Over all very good and easy to follow. Good explanations for each section of the course.
-
Elearning experience was excellent. Case studies, scenarios and knowledge checks were suitably detailed to really enhance the learning experience. User experience very good, course very well designed. Would not hesitate to recommend ComplyGuru courses.
-
The case studies were engaging and helped bring exposure to real world concepts. The clear definitions made it easier to grasp key concepts. The explanation of different RCA techniques was excellent, very detailed and easy to follow. Its a great course with strong content, definitely recommended.
Frequently Asked Questions