Advanced EU MDR Practitioner Training
Advanced EU MDR Practitioner Training (40hr) is for Quality & Regulatory Professionals who want comprehensive training on the European Medical Device Regulation (EU MDR 2017/745).
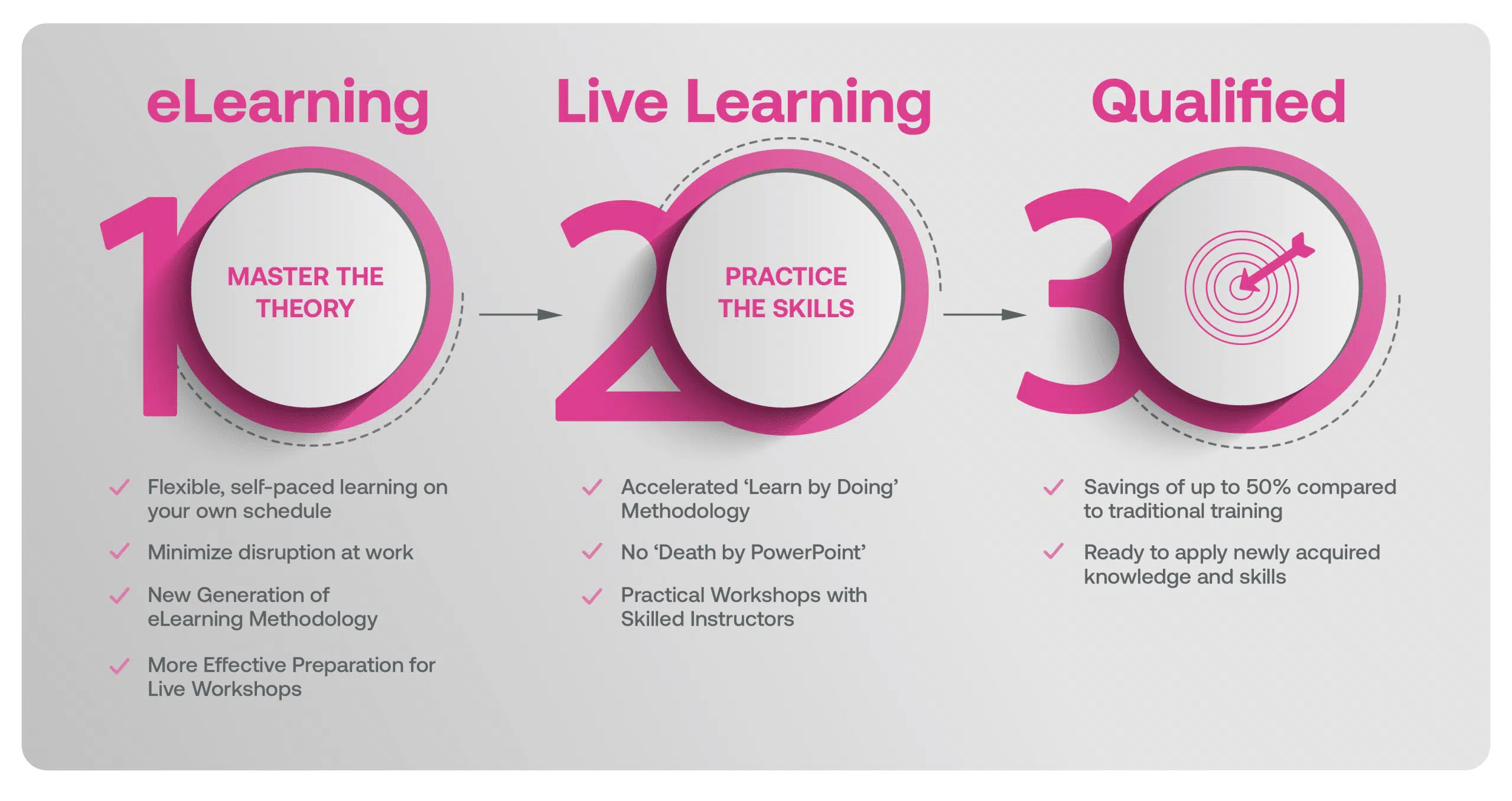
Our blended learning course is a hybrid approach where you first complete 24hrs of Practitioner eLearning modules on the underlying theory that better prepares you in advance of attending 2-days of Live Classes with an Instructor where Learners will work with case studies & scenarios that help deepen the understanding of the requirements in a real life context.




Download Brochure
View Sample Certificate of Achievement


Have 5 or more that need training?
We offer group discounts and are available for in-house (live or virtual) or tailored training in multiple formats.
Our Methodology
Blended Learning is Better Learning
A two-step methodology with eLearning modules that help you master the theory better preparing you for practical workshops that embed the skills.
Course Overview
Advanced EU MDR Practitioner
Explain the history, purpose, and structure of the EU MDR, and the key terminology used throughout the regulation
Identify the types of devices covered by the EU MDR and the rules for classifying these devices
Describe the obligations of the economic operators and the PRRC
Describe the General Safety & Performance Requirements and the key features of a risk management system based on ISO 14971
Describe the contents of the Technical Documentation and the requirements for Post Market Surveillance, Vigilance, and Clinical Data
Explain the Unique Device Identifier requirements and the relationship with EUDAMED
Introduction to the EU MDR
- History, Purpose & Structure of the EU MDR
- Key Terminology
- Key EU MDR changes
- EU MDR Timelines for Transition
Medical Devices covered by the EU MDR
- What is a device?
- Devices in & out of scope
- Relationship with other Directives
- Legacy Devices
Placing a Device on the Market
- Overview of Chapter II
- Articles 5-9
- Articles 10-16
- Manufacturer Obligations
- Authorized Representative
- Importer & Distributor Obligations
- Person Responsible for Regulatory Compliance (PRRC)
- Articles 17-20
- Articles 21-24
Device Classification
- Types of Risk Class
- Duration of Use
- Types of Body Contact
- Principles of Classification
- Continuous Use
- Classification Rules
- Non-Invasive Rules 1-4
- Invasive Rules 5-8
- Active Devices Rules 9-13
- Special Classification Rules
Routes to Conformity
- Overview of Conformity Assessment
- Annex IX Overview including Chapters 1-2
- QMS: Additional MDR Requirements
- Annex X Type Examination
- Annex XI Product Conformity Verification
- Conformity Assessment of Device Class
- Special Device Conformity
- Derogation from Device Conformity
- Notified Bodies
GSPR and Risk Management
- Chapter I General Requirements
- Risk Management
- Chapter II Design & Manufacture
- Chapter III Device Information
Clinical Evaluation
- Clinical Evaluation Introduction
- Article 61 Exemption and Update
- Annex XIV Part A: Clinical Evaluation and Part B: PMCF
- Summary of Safety & Clinical Performance (SSCP)
- Clinical Evidence for Legacy Devices
- Clinical Data Requirements
Post Market Surveillance and Vigilance
- Chapter VII Post Market Surveillance
- Vigilance Terminology & Reporting
- Trend Reporting
- Article 89
- Additional Considerations
- Medical Device Documentation
Technical Documentation, UDI and EUDAMED
- Annex II & III: Technical Documentation
- UDI Requirements & Timelines
- EUDAMED
- MDCG Guidance Documents
This course has been designed for those working in Quality Assurance and/or Regulatory Affairs who want to gain a better understanding of the EU Medical Device Regulation (EU MDR 2017/745), including:
- Quality Assurance Professionals
- Quality Engineers
- Research and Design Engineers
- Internal Auditors
- Quality Managers
- Manufacturing Engineers
- Regulatory professionals
Upon successful completion, each Learner shall receive a digital Certificate of Achievement within 1 business day.
Before completing this course, each Learner should have the following prior knowledge:
- A working knowledge of ISO 13485, which may be gained by completing Comply Guru’s ISO 13485 Foundation Course
- The relationship between ISO 13485 and the EU MDR 2017/745
- Commonly used quality management terms and definitions within ISO 13485 and ISO 9000
- A working knowledge of risk management principles related to the design of a medical device, through ISO 14971
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based) by the required deadline set in advance of the given workshop dates you are registered for (applies to blended format only)
- Fully attend the Live Instructor Workshops as 100% attendance is required
- Obtain 70% or higher in the graded assessments during the Live Instructor Workshops
- Complete a 1.5hr Final Examination* that is remotely invigilated (Live Proctored). Each Learner must obtain 70% or higher in the Final Examination which is completed securely online (under strict examination conditions).
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Watch and Learn More
About Our Advanced EU MDR Practitioner Training
Learn about how our Advanced EU MDR Training for Practitioners is leading the industry for QARA Professional Training.
Frequently Asked Questions