EU IVDR Lead Auditor Training for the European In Vitro Diagnostic Regulation (2017/746)
EU IVDR Lead Auditor Training to provide learners with the knowledge and skills required to perform audits of Medical Device Quality Management Systems (MD-QMS) against the European Union’s In Vitro Diagnostic Regulation (EU IVDR 2017/746) in accordance with ISO 19011 and ISO 17021, as applicable.
During live workshops, our experienced Instructors will use practical training methods with real-world examples, case studies, facilitated exercises and group discussions. Upon completion, Learners will be equipped to make an instant impact with their newly acquired knowledge and skills.




Download Brochure
View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain a Globally Recognized EU IVDR Auditor Qualification
Advance your knowledge and enjoy more flexibility & learning effectiveness with Comply Guru.

Register 3 + Get 1 Free

Course Overview
EU IVDR Lead Auditor Training
Explain the background and purpose of a medical device quality management system (MD-QMS) in the context of EU IVDR 2017/746 and confirm links with ISO 13485.
Explain the role and responsibilities of an auditor to plan, conduct, and report nonconformities for a quality management system audit in accordance with ISO 19011/ISO 17021 that uses the EU IVDR 2017/746 requirements as the audit criteria.
Plan for and conduct an audit of a medical device quality management system to establish compliance with the EU IVDR 2017/746 in accordance with ISO 19011/ISO 17021 as applicable and report on any nonconformities.
Upon successful completion, each Learner shall receive a digital Certificate of Achievement.
Prior to attending this course, learners are expected to have the following prior knowledge:
EU IVDR
- A good understanding of the EU IVDR (2017/746) requirements and their application is required for this course. This may be gained by successfully completing our EU-IVDR 2017/746 Practitioner course. A copy of the training certificate will be requested for verification purposes as part of acceptance onto the course.
Please note: if no recognized certificate is provided, additional information & a pre-course test may be required to verify knowledge of the requirements in order to be accepted onto the course.
ISO 13485
- Must have successfully completed a CQI and IRCA Certified MD-QMS ISO 13485 Lead Auditor course or equivalent. A copy of the training certificate will be requested for verification purposes as part of acceptance onto the course.
Medical device management systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
- The process approach used in quality management.
- A working knowledge of risk-management principles related to the design of a medical device, through ISO 14971.
To successfully complete this course, each Learner will need to:
- Fully attend the Instructor Workshops as 100% attendance is required.
- Obtain 70% or higher in the graded assessments during the Instructor Workshops
- Pass the end of course final assessment.
For the live workshops during a virtual delivery, we utilise both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
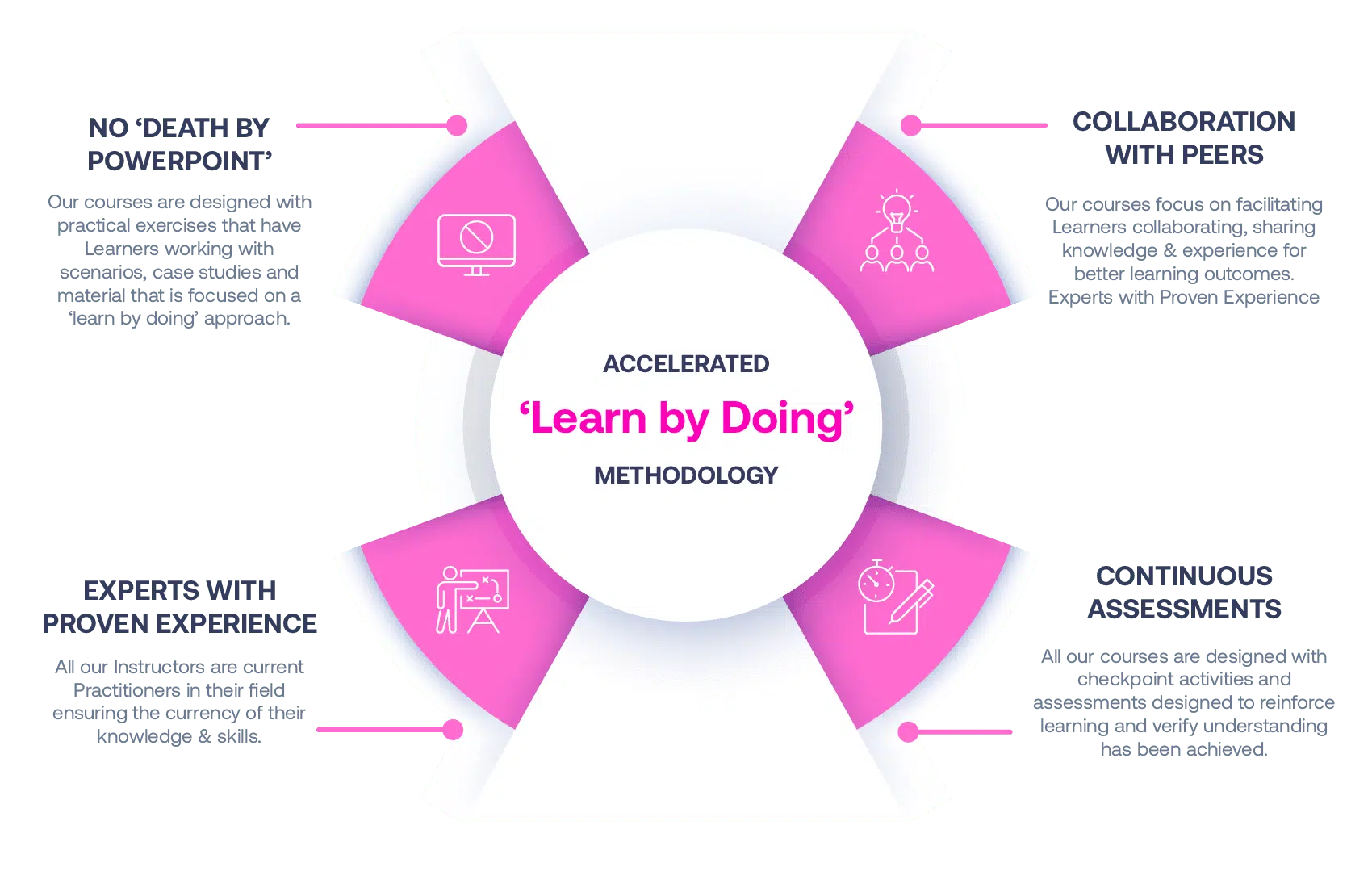
Our Methodology
No More Death By PowerPoint
Our courses are designed with practical exercises that have Learners working with scenarios, case studies and material that is focused on a ‘learn by doing’ approach.
Course Structure Explained
Detailed Breakdown & Agenda
Attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| 09:00am |
Practical Workshop Audit Objective, Scope & Criteria |
| 10:00am | Break |
| 10:10am |
Practical Workshop Device Classification |
| 11:10am | Break |
| 11:25am | Practical Workshop Device Conformity Assessment Routes |
| 12:30pm | Lunch |
| 1:15pm | Audit Lifecycle Practical Workshop Simulated Case Study |
| 2:15pm | Break |
| 2:25pm | Practical Workshop Simulated Case Study, continued |
| 3:30pm | Break |
| 3:45pm | Practical Workshop Simulated Case Study, continued Exam Preparation & Guidance
|
| 5:00pm | End of Day 1 Wrap Up |
| Time | Topic |
|---|---|
| 09:00am | Day 1 Recap |
| 10:00am | Break |
| 10:10am | Practical Workshop Reporting Audit Findings |
| 11:10am | Break |
| 11:25am | Practical Workshop Reporting Audit Findings, continued |
| 12:30pm | Lunch |
| 1:15pm | Practical Workshop Report and Close |
| 2:15pm | Break |
| 2:25pm | Practical Workshop Audit Follow Up |
| 3:45pm |
|
| 5:00pm | End of Day 2 Wrap Up |
| Time | Topic |
|---|---|
| 9:00am |
|
| 10:00am | Certification Exam (via eAssessment) |
Watch and Learn More
About our EU IVDR Auditor Training
Learn about the benefits and key features of Comply Guru’s Instructor led Learning.
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.8
Average Rating
5 global ratings
-
Challenging subject but delivered in a structured and light hearted way which makes the content much more manageable to digest and get through
-
Really enjoyed the course, interactive team were sessions were great and excellent practice of mock IVDR auditing. Michelle was excellent and is a breath of fresh air bringing a motivational approach to a heavy topic.
-
I've always found the training to be very effective. Michelle is a great instructor.
-
It was brilliant training with amazing instructor Michelle who always gives a lot advice and pointers which are useful in real world scenarios