FDA QMSR Transition Training for Practitioners and Auditors
FDA QMSR Transition Training is for Quality and Regulatory Professionals who are already familiar with ISO 13485 and FDA’s current Quality System Regulation (QSR) and want to upskill their knowledge of the key differences, implementation impacts and compliance expectations being introduced under the new Quality Management System Regulation (QMSR).
An experienced Instructor will facilitate practical workshops that focus on the real-world implications of the shift from QSR to QMSR offering guided exercises, case studies, examples and group discussions.
Explained: The difference between 16hr FDA QMSR Requirements and 8hr FDA Transition Training
The main difference is that Comply Guru’s 16hr FDA QMSR Requirements training includes additional coverage of ISO 13485 requirements (including similarities and differences vs the QMSR), whereas our 8hr FDA QMSR Transition training is a slimmed down version focusing exclusively on the QSR to QMSR changes.





Download Brochure
View Sample Certificate of Completion


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain a Recognized Qualification
Advance your knowledge and upskill with one of our Instructors.

Course Overview
FDA QMSR Transition Training
On completion of this course, successful Learners will have the practical knowledge needed to:
- Explain the rationale behind the FDA’s move from QSR to QMSR
- Identify and Analyze the key differences between QSR and QMSR
- Understand how ISO 13485 is being integrated into FDA Regulations
- Evaluate the impact of QMSR on currently Quality Systems and Documentation
- Develop an action plan for transitioning to QMSR
Prior to attending this course, learners are expected to have the following prior knowledge:
FDA QSR 21 CFR Part 820
- Familiarity with the FDA QSR 21 CFR Part 820 & related requirements.
ISO 13485
- Familiarity with ISO 13485:2016 requirements.
- Quality Professionals
- Quality Engineers
- Regulatory Professionals
Successful completion will entitle each Learner to receive a digital Certificate of Completion within 1 business day.
In order to successfully complete this course, each Learner will need to:
- Fully attend the Live Instructor Workshop as 100% attendance is required
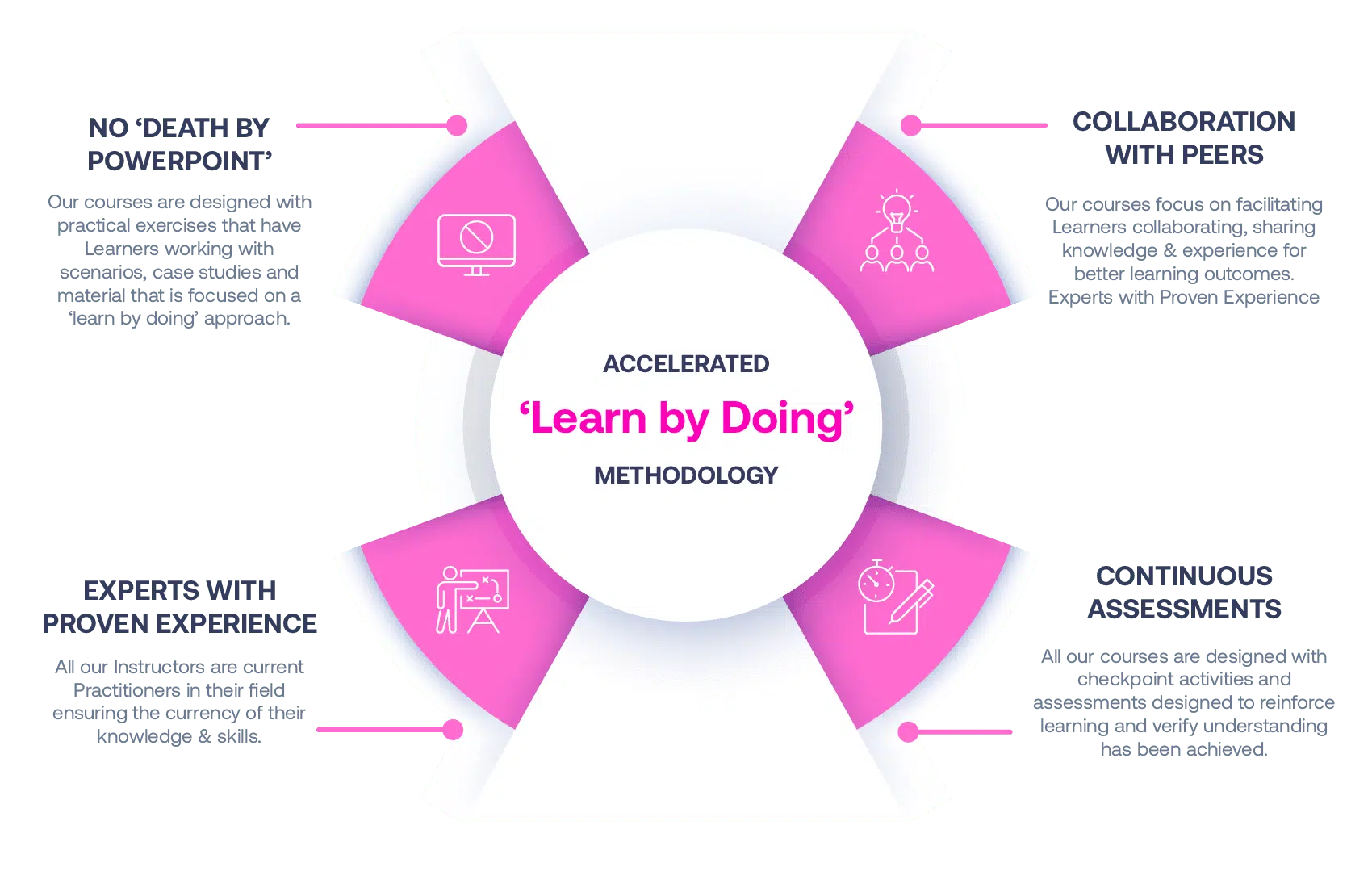
Our Methodology
No More Death By PowerPoint
Our courses are designed with practical exercises that have Learners working with scenarios, case studies and material that is focused on a ‘learn by doing’ approach.
Course Structure Explained
Detailed Breakdown & Agenda
Attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| #1 | Introduction and Context
Group Discussion “What are you biggest concerns or questions?” |
| #2 | Structural Comparison – QSR vs QMSR
Practical Workshop Mapping Exercise |
| #3 | Key Difference in Requirements
Practical Workshop Gap Impact Analysis |
| #4 | Implementation Challenges and Solutions
Practical Workshop QMSR Transition Scenarios |
| #5 | FDA Inspection Readiness under QMSR
|
| #6 | Wrap-Up and Q&A
|
Our Experts
Meet The Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About our FDA QMSR Transition Training
Learn about the benefits and key features of Comply Guru’s Instructor led Learning.
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4
Average Rating
1 global ratings
-
Michelle was excellent as always and very patient.
Frequently Asked Questions