Good Documentation Practices (GDP) in Medical Devices Training
This introductory course provides a practical overview of Good Documentation Practices (GDP) in the medical device industry. GDP is the foundation of regulatory compliance and quality ensuring that records are accurate, complete, legible and traceable.
Through real-world examples, you’ll learn the “why” and “how” of GDP, including best practices for creating, handling, and maintaining documents and records that support regulatory inspections, audits, and day-to-day operations.
Our eLearning is a highly interactive way to learn at a time & a pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.





Download Brochure
Have 5 or more that need training?
We offer group discounts and are available for in-house (live or virtual) or tailored training in multiple formats.
Course Overview
Good Documentation Practices (GDP) in Medical Devices
At the end of this short course, successful learners will be able to:
- Explain what GDP is and why it is critical for regulatory compliance in the medical device industry
- Apply the ALCOA+ principles
- Recognize common errors and pitfalls in documentation and how to avoid them
- Demonstrate correct practices for creating, reviewing, approving, and storing documentation
- Understand the regulatory expectations of authorities
This course is for individuals or organizations who want to understand Good Documentation Practices (GDP) in the medical device industry.
Successful completion of the course examination will entitle each Learner to receive a digital Certificate of Completion.
In order to successfully complete this course, each Learner will need to:
- Complete all eLearning modules and obtain 70% or higher in the final assessment (MCQ-based)
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
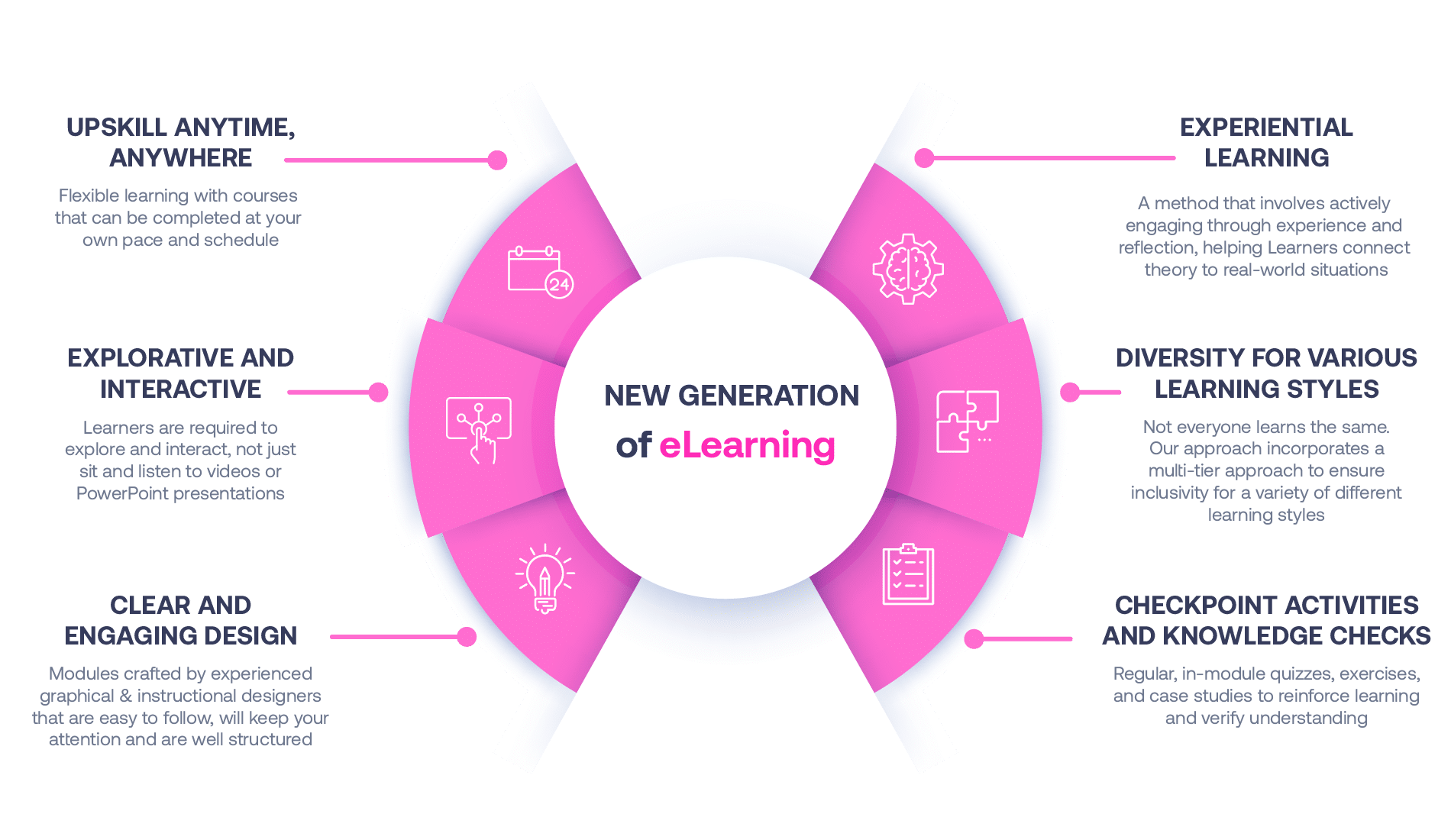
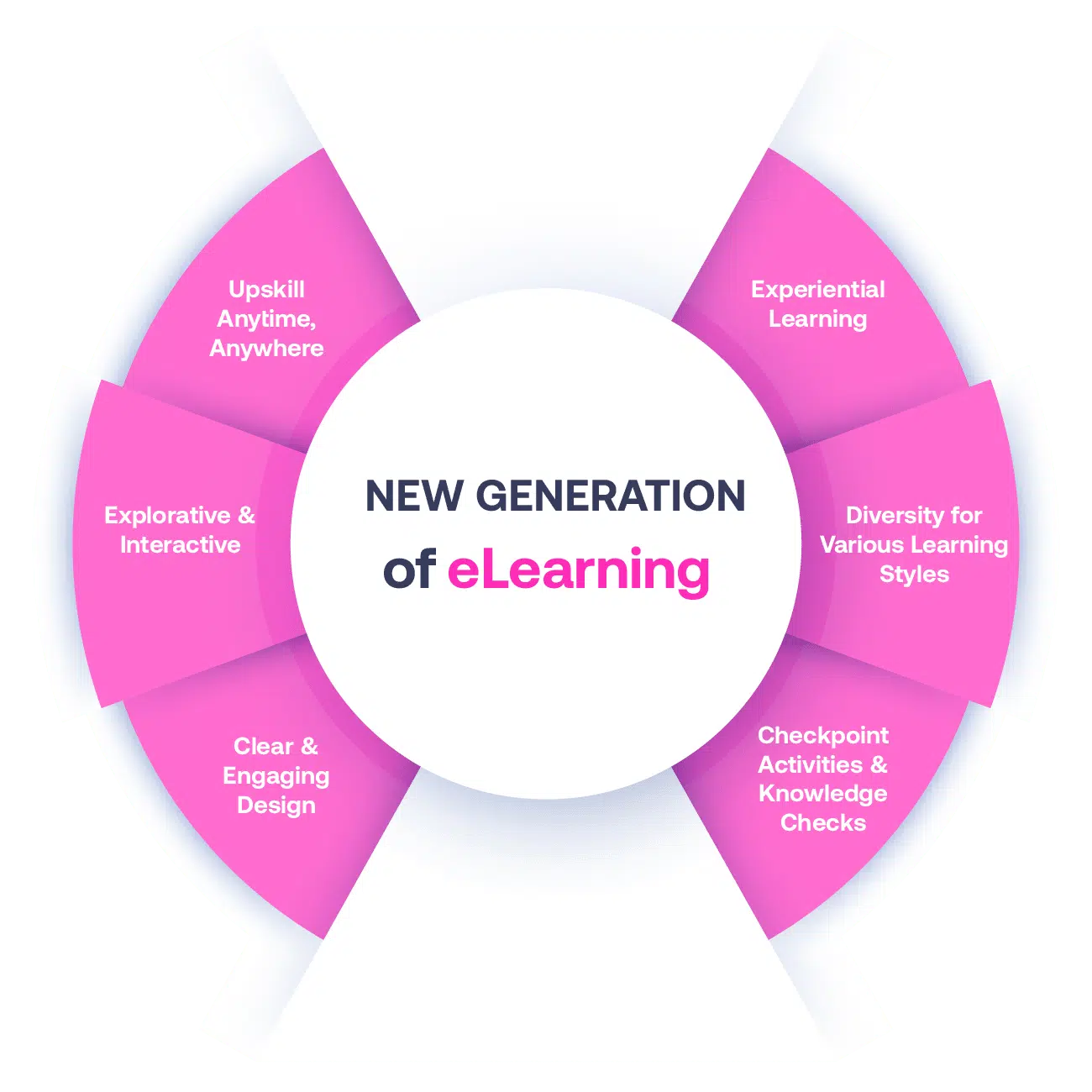
Our Methodology
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Our Experts
Meet The People Behind The Course
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About our eLearning
Learn about how our eLearning is leading the industry for innovation through online learning