TRUSTED & ACCREDITED
Comply Guru offers globally recognized qualifications that are more accessible & flexible to help you get the training you need when you need it.



Browse Our Top Courses
We offer transformational training for the modern workforce that does not compromise on learning effectiveness.

FEATURED CUSTOMER

FEATURED CUSTOMER

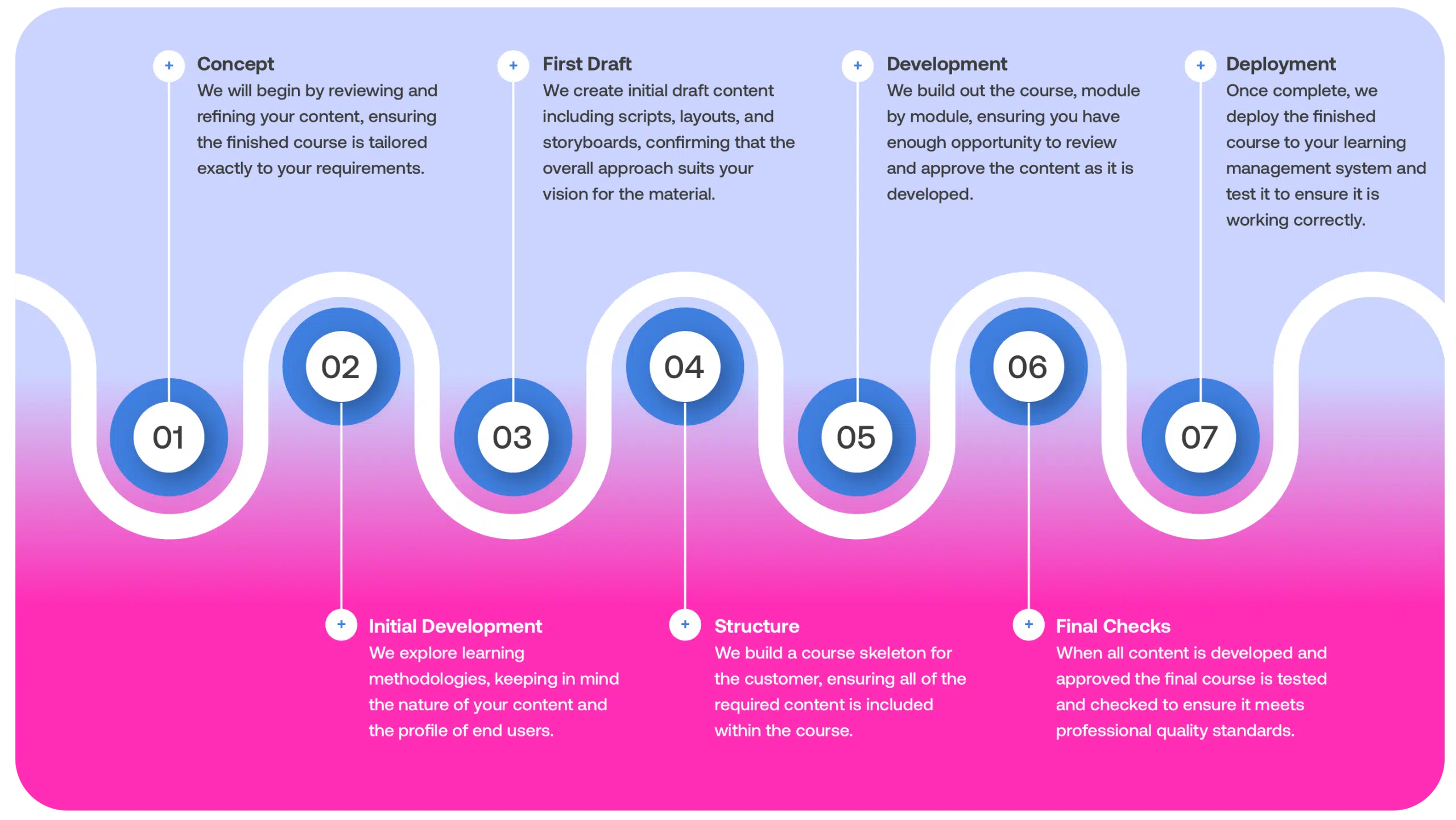
Contract Auditing
Experienced Professionals to Ensure your Organization is Fully Prepared
Comply Guru offers a comprehensive range of audit services that span across a diverse spectrum of industry standards and regulations.
Whether you’re in the Life Sciences, manufacturing, services, or any other industry, we tailor our services to your unique needs.
Read, Watch & Discover More
Our team has you covered with expert insights & updates to stay on top of the latest updates, trends, and changes in the industry.



Download Our Current Training Guide