Advanced Risk Practitioner (ISO 14971) for Medical Devices
Advanced Risk Practitioner Training (Medical Devices) is for anyone who wants both a comprehensive overview of the international standard for risk management for medical devices, and to acquire the best practice skills to readily apply the knowledge and tools successfully within their organization.
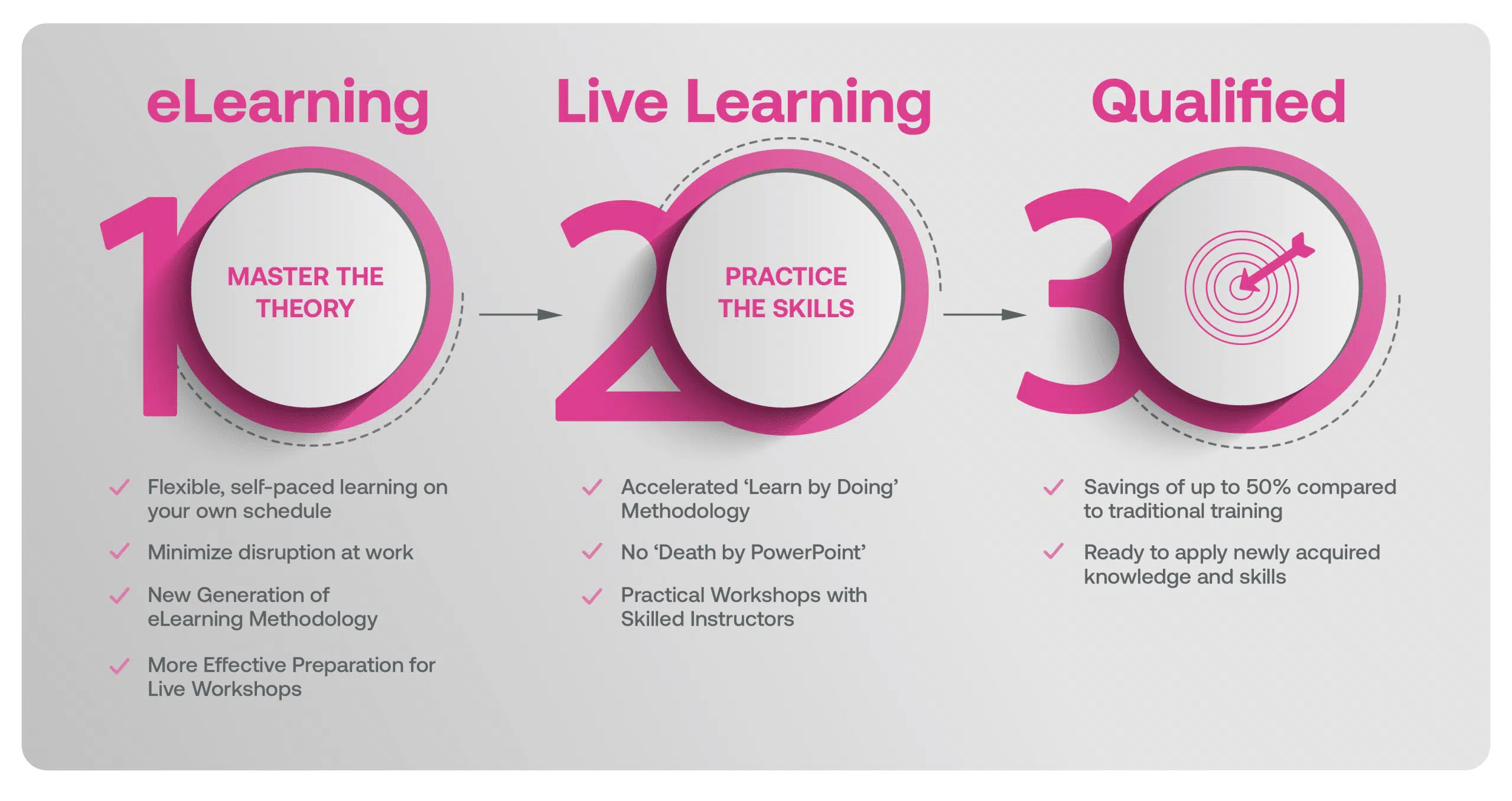
Our blended learning course is a hybrid approach where you first complete eLearning modules on the underlying theory that better prepares you in advance of attending Live Classes with an Instructor where Learners will work with case studies & scenarios that help deepen the understanding of the requirements, and applying the tools & techniques in a real life context.
The Regulatory Affairs Professionals Society (RAPS) recognize this course offering global recognition for successful participants and RAPS credits for members.





View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain an Recognized Risk Practitioner Qualification
Advance your knowledge and enjoy more flexibility & learning effectiveness with Comply Guru.

Course Overview
Advanced Risk Practitioner (ISO 14971) Training
On completion of this course, successful Learners will have the practical knowledge to support their organization and apply the techniques needed to:
- Explain the purpose and scope of ISO 14971
- Outline the steps to establish, document and maintain a risk management process
- Analyze risk evaluation techniques and criteria in compliance with ISO 14971
- Apply risk control measures to reduce residual risks effectively
- Conduct post-market surveillance and continuous risk monitoring
This course is aimed at anyone working in the medical device sector that wants to master risk management based on ISO 14971, including:
- Design & Development personnel
- Quality / Engineering / Technical / Production personnel
- Regulatory affairs
- Internal / Lead / Supplier Auditors
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (12).
Upon successful completion, each Learner shall receive a digital Certificate of Achievement within 1 business day.
Before completing this course, each Learner is recommended to have the following prior knowledge:
- The fundamental concepts and principles of risk management as it applies to medical devices
- The commonly used risk terms and definitions (see ISO 14971)
- A working knowledge of ISO 13485, which may be gained by completing Comply Guru’s ISO 13485 Foundation Course
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessment (MCQ-based)
- Fully attend the Live Instructor Workshops as 100% attendance is required
- Obtain 70% or higher in the graded assessments during the Live Instructor Workshops
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
Blended Learning is Better Learning
A two-step methodology with eLearning modules that help you master the theory better preparing you for practical workshops that embed the skills.
Course Structure Explained
Detailed Breakdown & Agenda
Learners first complete interactive eLearning modules to grasp the underlying theory, then attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| 75mins eLearning | Module 1: Introduction to Risk Management
|
| 75mins eLearning | Module 2: Risk Management Planning
Case Study! Elements of a Risk Acceptability Policy |
| 90mins eLearning | Module 3: Risk Assessment
Case Study! Safety Characteristics Case Study! Hazard Identification and Sequence of Events |
| 60mins eLearning | Module 4: Risk Control
Case Study! Benefit Risk Analysis Case Study! Overall Residual Risk |
| 60mins eLearning | Module 5: Risk Management Review and Update
Case Study! Post-Market Surveillance and Risk |
| 90mins eLearning | Module 6: ISO/TR 24971
|
| 40mins | Eligibility for Live Workshop Exam
|
| Time | Topic |
|---|---|
| #1 | ISO 14971 eLearning Recap
Practical Workshop! Integrating Risk Assessment Tools into the ISO 14971 Process |
| #2 | Risk Management Process Overview
Practical Workshop! Building a Comprehensive Risk Management File (RMF) |
| #3 | Risk Analysis
Practical Workshop! Using FMEA for Risk Identification and Estimation |
| #4 | Risk Evaluation
Practical Workshop! Setting and Applying Risk Acceptance Criteria |
| #5 | Risk Control
Practical Workshop! Verifying the effectiveness of Risk Control |
| #6 | Production and Post-Production Activities
Practical Workshop! The Role of Post-market Surveillance in Risk Management |
Watch and Learn More
About our Advanced Risk Practitioner Training
Learn about how our Training is leading the industry for innovation & learning effectiveness
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.4
Average Rating
7 global ratings
-
The training is highly practical and well structured.
-
My experience of training with Comply Guru and Michelle Keane was excellent, in-depth, and provided exactly what the course described. Michelle is extremely knowledgeable and made the course content enjoyable and very worthwhile
-
Great learning method based on scenarios in real life. Perfect organisation and support. Amazing trainer Michelle, very knowledgeable and able to create a perfect relaxed training environment.
-
Really appreciate the blended learning, really good learning environment.
-
As always, Michelle was brilliant
Frequently Asked Questions