ISO 13485 Requirements for Practitioners and Auditors
ISO 13485 Requirements for Practitioners and Auditors Training is for anyone looking to gain a comprehensive understanding of the core requirements of ISO 13485 and this highly interactive course can be completed anytime, anywhere (24/7).
What makes Comply Guru’s eLearning different from competitors is we instructionally design modules based on engaging, applied learning with real-world examples and case studies. Our online training is a highly interactive way to learn at a time & pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.
This course is an internationally accredited credential. This course is certified by the Chartered Quality Institute (CQI) and International Registrar of Certificated Auditors (IRCA) under course number 2589. This course is also recognized by the Regulatory Affairs Professionals Society (RAPS) where members will be eligible to receive 8 credits by completing this course.





View Sample Certificate of Completion


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Course Overview
ISO 13485 Requirements Training
Explain the purpose of a medical device quality management system, of medical device quality management systems standards, and the business benefits of improved performance given by an effectively implemented medical device quality management system
Outline the structure and content of ISO 13485 and its relationship with ISO 9001 and IMDRF individual country regulatory documents
Explain the specific quality management-related requirements of ISO 13485
This course is targeted at:
-
- Quality Managers
- Quality Professionals/Technicians
- Quality Engineers
- Procurement Personnel
- Financial Personnel
- Document Controllers
- Internal Auditors
- Owner Managers
- Implementation Members
Upon successful completion, each Learner shall receive two digital Certificates of Completion.
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (8).
The first certificate is issued automatically by Comply Guru within 1hr of the course completion that is recognized by the Regulatory Affairs Professionals Society (RAPS).
It is recommended that each Learner should have the following prior knowledge before completing this course:
- The Plan, Do, Check, Act (PDCA) cycle
- The core elements of a management system and the interrelationship between top management responsibility, policy, objectives, planning, implementation, measurement, review, and continual improvement.
- The fundamental concepts and commonly used quality management terms and definitions
Fluency in written and spoken English
- For participants whose first language is not English, we recommend a minimum English language competency of IELTS 5.5 (or equivalent) for successful completion of the program. This is not assessed by Comply Guru in advance & each participant must self-assess their competency.
Copy of ISO 13485 (International Standard)
- It is recommended that each Learner should have a copy of ISO 13485:2016 (International Standard) to reference while completing this course. This is not provided by Comply Guru and without it, this course will be challenging to complete successfully.
In order to successfully complete this course, each Learner will need to:
- Complete the course modules and obtain 70% or higher in the final assessment (MCQ-based)
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
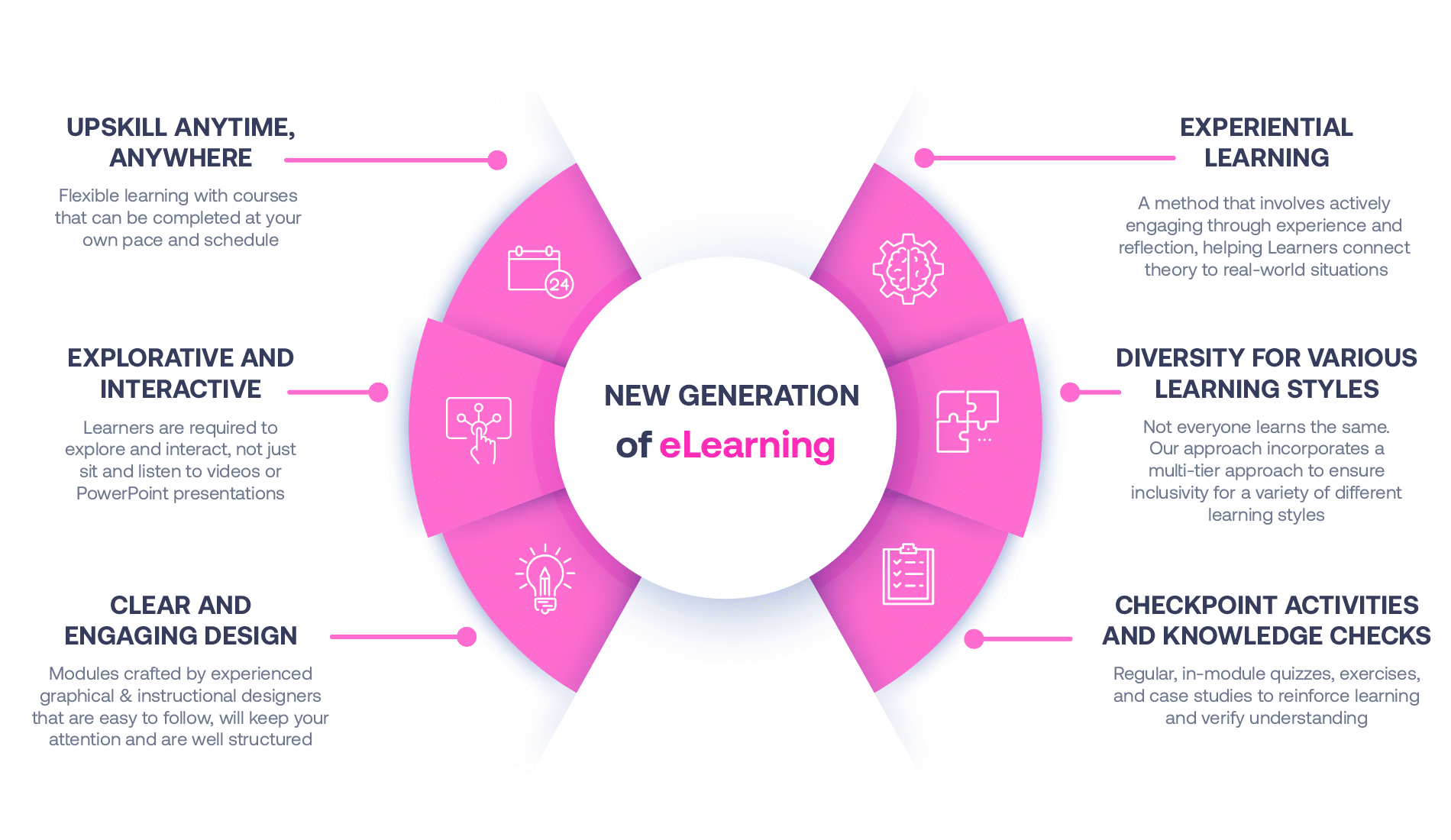
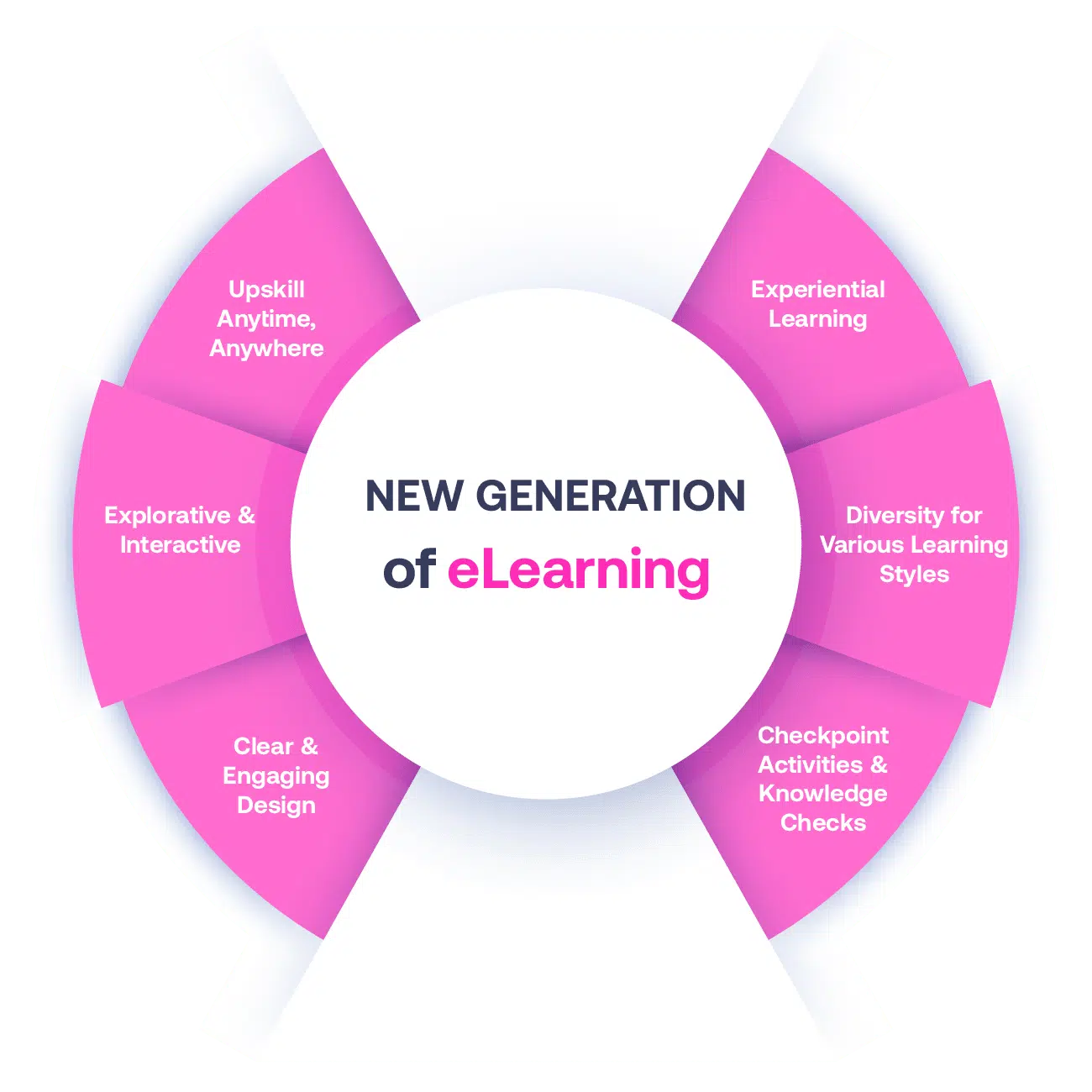
Our Methodology
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Course Structure Explained
eLearning Module Breakdown & Timings
An interactive, engaging eLearning experience that you can complete Anytime, Anywhere.
| Time | Topic |
|---|---|
| 70mins eLearning | Module 1 – Overview of ISO 13485
Case Study! Risk Management |
| 110mins eLearning | Module 2 – Establishing a Quality Management System
Case Study! Software Validation Case Study! Record Control |
| 65mins eLearning | Module 3 – Management Responsibility
Case Study! Management Review |
| 80mins eLearning | Module 4 – Resource Management
Case Study! Training and Document Updates Case Study! Work Environment and Contamination Control |
| 95mins eLearning | Module 5 – Product Realization, Part 1
Case Study! Customer Related Processes |
| 70mins eLearning | Module 6 – Product Realization, Part 2
Case Study! Purchasing Controls |
| 70mins eLearning | Module 7 – Measurement, Analysis, and Improvement
Case Study! Complaint Handling Case Study! Internal Audits |
| 45mins | Certification Exam
|
Watch and Learn More
About Our Requirements Training
Learn about how our eLearning is leading the industry for innovation & learning effectiveness
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.5
Average Rating
540 global ratings
-
The structure of the modules, the knowledge checks, scenarios were very well structured. I gained a high level of understanding and my motivation was on a high level during the whole training. I can recommend.
-
I found the training experience to be very effective, especially on the sections where there were case studies & scenarios hat you had to identify a course of action to take. I found this element to be very beneficial within the course.
-
Found the online content easy to navigate.
-
Finished Comply Gurus self paced ISO 13485 course. It is a simple to follow, clear and engaging course. Good way to learn the basics with no fuss.
-
Knowledgable
Frequently Asked Questions