ISO 14971 Requirements for Practitioners and Auditors
ISO 14971 Requirements Training for Practitioners and Auditors that provides a comprehensive overview of the international standard for risk management for medical devices. This course has been specifically designed for Quality & Regulatory Professionals in the Medical Device Industry.
What makes Comply Guru’s eLearning different from competitors is we instructionally design modules based on engaging, applied learning with real-world examples and case studies. Our online training is a highly interactive way to learn at a time & pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.
This course is an internationally accredited credential. CQI and IRCA certify this course (No. 2415) and the Regulatory Affairs Professionals Society (RAPS) recognize this course offering global recognition for successful participants.





View Sample Certificate of Completion


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Course Overview
ISO 14971 Requirements Training
Explain the history, purpose, and structure of ISO 14971 and its relationship to ISO 13485 and the EU & US FDA regulations
Define the key terminology used throughout ISO 14971
Describe the elements of an effective risk management process
Describe the different techniques that support risk analysis
Explain the requirements for updating the risk assessment based on information collected and reviewed
Outline the role of ISO TR 24971 in risk management
This course is aimed at anyone working in the medical device sector that wants to gain an understanding of risk management based on ISO 14971, including:
- Design & Development personnel
- Quality / Engineering / Technical / Production personnel
- Regulatory affairs
- Internal / Lead / Supplier Auditors
Upon successful completion, each Learner shall receive two digital Certificates of Completion.
The first certificate is issued automatically by Comply Guru within 1hr of the course completion.
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (10).
CQI and IRCA certify this course (No. 2415) under the course title: FD133: MD-QMS ISO 14971:2019 Foundation Training and Learners shall also receive a second training certificate recognized by CQI and IRCA within 2 business days.
Before completing this course, each Learner is recommended to have the following prior knowledge:
- The fundamental concepts and principles of risk management as it applies to medical devices
- The commonly used risk terms and definitions (see ISO 14971)
- A working knowledge of ISO 13485, which may be gained by completing Comply Guru’s ISO 13485 Foundation Course
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessment (MCQ-based)
There are recommended requirements for each Learner in wishing to complete any of our eLearning modules. In our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
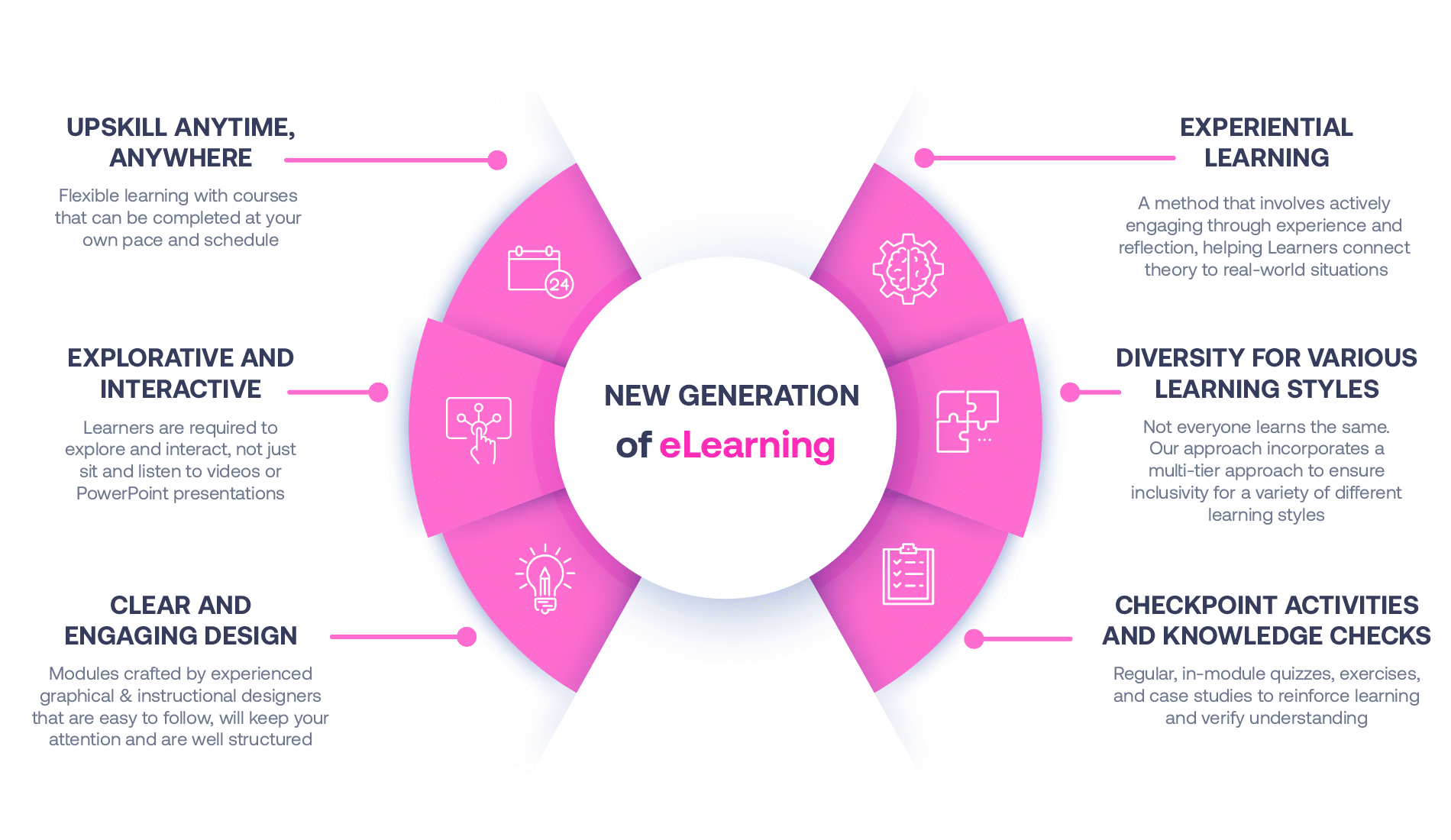
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Course Structure Explained
eLearning Module Breakdown & Timings
An interactive, engaging eLearning experience that you can complete Anytime, Anywhere.
| Time | Topic |
|---|---|
| 75mins eLearning | Module 1: Introduction to Risk Management
|
| 90mins eLearning | Module 2: Risk Management Planning
Case Study! Elements of a Risk Acceptability Policy |
| 120mins eLearning | Module 3: Risk Assessment
Case Study! Safety Characteristics Case Study! Hazard Identification and Sequence of Events |
| 90mins eLearning | Module 4: Risk Control
Case Study! Benefit Risk Analysis Case Study! Overall Residual Risk |
| 75mins eLearning | Module 5: Risk Management Review and Update
Case Study! Post-Market Surveillance and Risk |
| 90mins eLearning | Module 6: ISO/TR 24971
|
| 40mins | Certification Exam
|
Watch and Learn More
About Our Requirements Training
Learn about how our Globally Recognized ISO 14971 Training is leading the industry for innovation through online learning
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.4
Average Rating
467 global ratings
-
very informative
-
This course reaffirmed much of my historical knowledge This course is valuable asset along with the other QMS processes
-
good learning experience
-
Good content, a lot of material covered, deeper understanding of the standards and its application in medial device. I thought the length of the course was perfect and the time to complete was very manageable. The interactive elements really helped me to grasp the deeper understanding.
-
The content was well structured and detailed. However some aspects felt a bit repetitive and could have been made more concise. I liked the tests at the end of each module which enabled me to consolidate what I had learned and the case studies helped to translate the theory to real world scenarios
Frequently Asked Questions