Person Responsible for Regulatory Compliance (PRRC) Training
Person Responsible for Regulatory Compliance (PRRC) Training will provide learners with clarity on the role and responsibilities of the Person Responsible for Regulatory Compliance (PRRC) introduced under Article 15 of the Medical Device Regulation (MDR) and In Vitro Diagnostics Regulation (IVDR) and provide details on the qualifications and liabilities that exist for PRRCs.
What makes Comply Guru’s eLearning different from competitors is we instructionally design modules based on engaging, applied learning with real-world examples and case studies. Our online training is a highly interactive way to learn at a time & pace that works best for you, making it easier to get the training you need while balancing a busy home or work life.
This course is an internationally accredited credential. The Regulatory Affairs Professionals Society (RAPS) recognize this course offering global recognition for successful participants and RAPS credits for members.





View Sample Certificate of Completion


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Course Overview
Person Responsible for Regulatory Compliance (PRRC) Training
Establish an understanding as to why the role of the PRRC was introduced in the MDR and IVDR
Identify the qualifications and relevant experience required to become a PRRC, how to demonstrate them and document the qualifications and relevant experience
Discuss and implement the elements involved in the appointment of the PRRC (appointment letter, job description)
Recognize the requirements of appointing a PRRC (Internal PRRC vs Outsourced PRRC)
Understand the responsibilities of the PRRC of a manufacturer and the Authorized Representative
Comprehend the requirements of registration of the PRRC on EUDAMED
Understand the integration and interaction of the PRRC into the organization and the impact of the PRRC on the Organization’s Quality Management System (QMS)
Understand the Liability of the PRRC
- Quality Assurance Professionals
- Quality Engineers
- Regulatory Professionals
- Internal Auditors
- Lead Auditors
- Management Representatives
- Top Management
The Regulatory Affairs Professionals Society has approved Comply Guru (No. 1007) and recognizes this course where members will be eligible for the stated number of RAPS credits (5).
Successful completion will entitle each Learner to receive a digital Certificate of Completion within 1 business day.
In order to successfully complete this course, each Learner will need to:
- Complete all eLearning modules and obtain 70% or higher in the final assessment (MCQ-based)
Prior to attending this course, learners must be informed that they are expected to have the following prior knowledge:
EU MDR or IVDR
- A good understanding of the EU MDR (2017/745) and/or EU IVDR (2017/746) requirements and their application.
ISO 13485
- Must have experience of working with ISO13485
Medical device management systems
Knowledge of the following quality management principles and concepts:
- The relationship between ISO 13485 and applicable international regulatory requirements for medical devices.
- The process approach used in quality management.
If you are completing this training, the following applies:
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
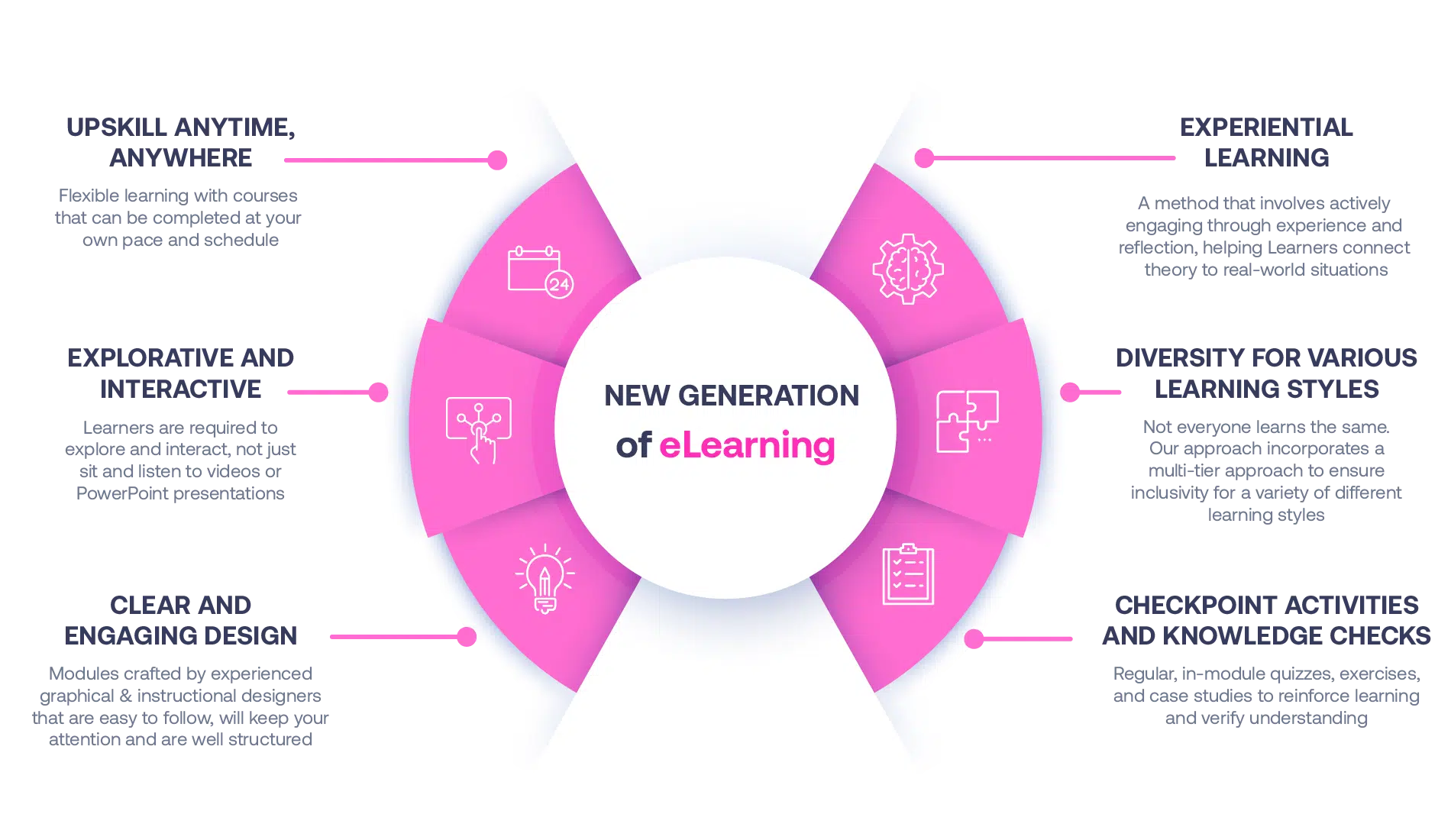
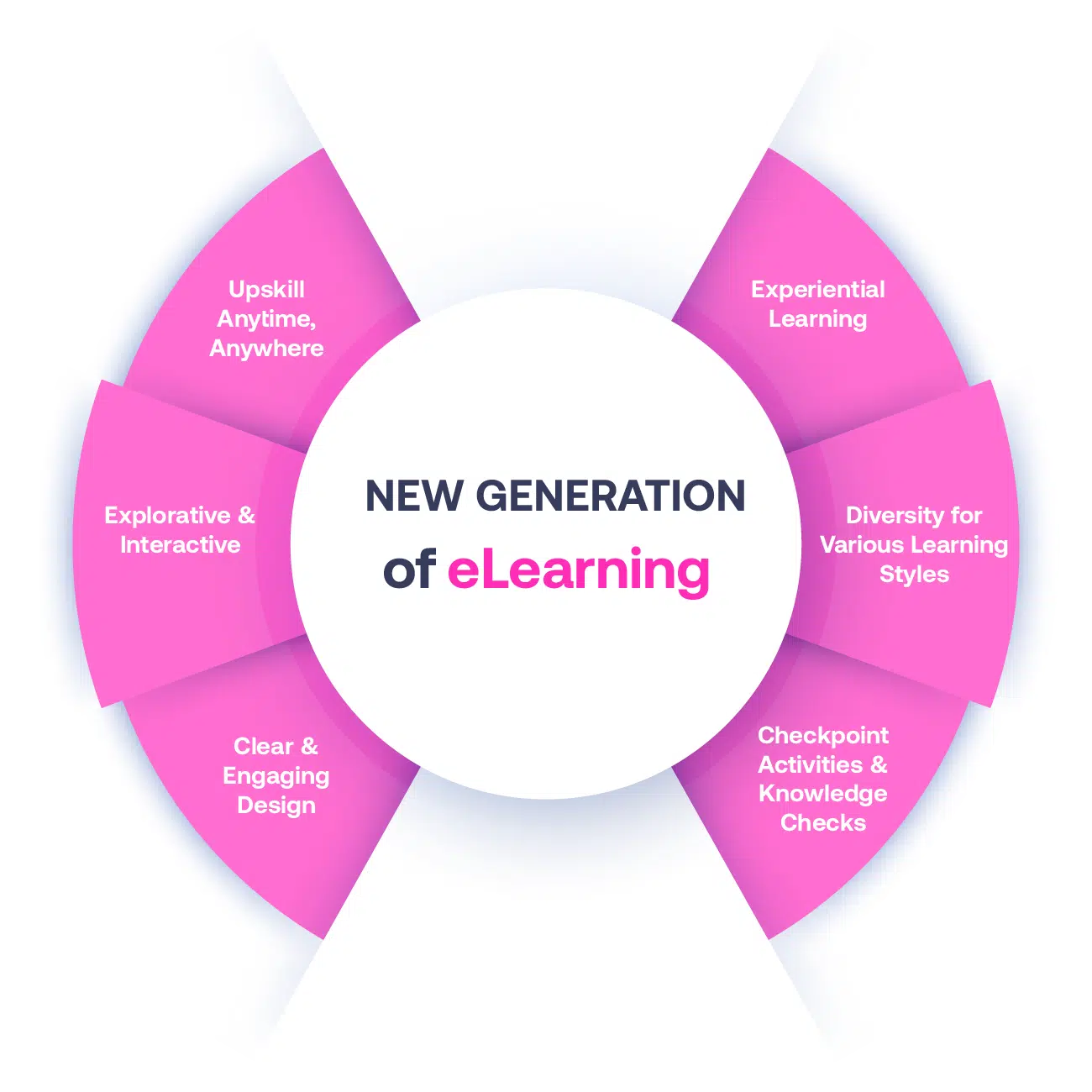
Our Methodology
Not all eLearning is the same
Most other providers offer online training that is one-dimensional utilizing either Videos or PowerPoint Presentations. That is not effective training. We deploy a multi-layered methodology that offers you a New Generation of eLearning.
Course Structure Explained
eLearning Module Breakdown & Timings
An interactive, engaging eLearning experience that you can complete Anytime, Anywhere.
| Time | Topic |
|---|---|
| 45mins eLearning | Module 1: The Introduction of the role of the PRRC in EU-MDR and EU-IVDR
|
| 45mins eLearning | Module 2: The qualifications and relevant experience required to become a PRRC
|
| 60mins eLearning | Module 3: The appointment of the PRRC
|
| 60mins eLearning | Module 4: Understand the responsibilities of the PRRC of a Manufacturer and the Authorized Representative
|
| 90mins eLearning | Module 5: Integration of the PRRC into the organization and the impact of the PRRC on the Organization’s Quality Management System (QMS)
|
| 30mins | Certification Exam
|
Our Experts
Meet The People Behind The Course
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About our PRRC Training
Learn about how our training is leading the industry for innovation through online learning.
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.5
Average Rating
27 global ratings
-
Good PRRC overview course in general
-
I really enjoyed the course, and the structure of the content was well suited for my learning needs.
-
Very detailed content with a good mixture of both listening and reading elements to ensure full understanding.
-
Was clear and easy to understand. Module flow and usability was great.
-
overall i found the course intuitive and easy to use which made the learning experience more engaging
Frequently Asked Questions