ISO 13485 Internal Auditor Training for Medical Device Quality Management Systems (MD-QMS)
ISO 13485 Internal Auditor Training is for anyone that wants to be part of the team that conducts internal audits of part of their Medical Device Quality Management System (MD-QMS) based on ISO 13485.
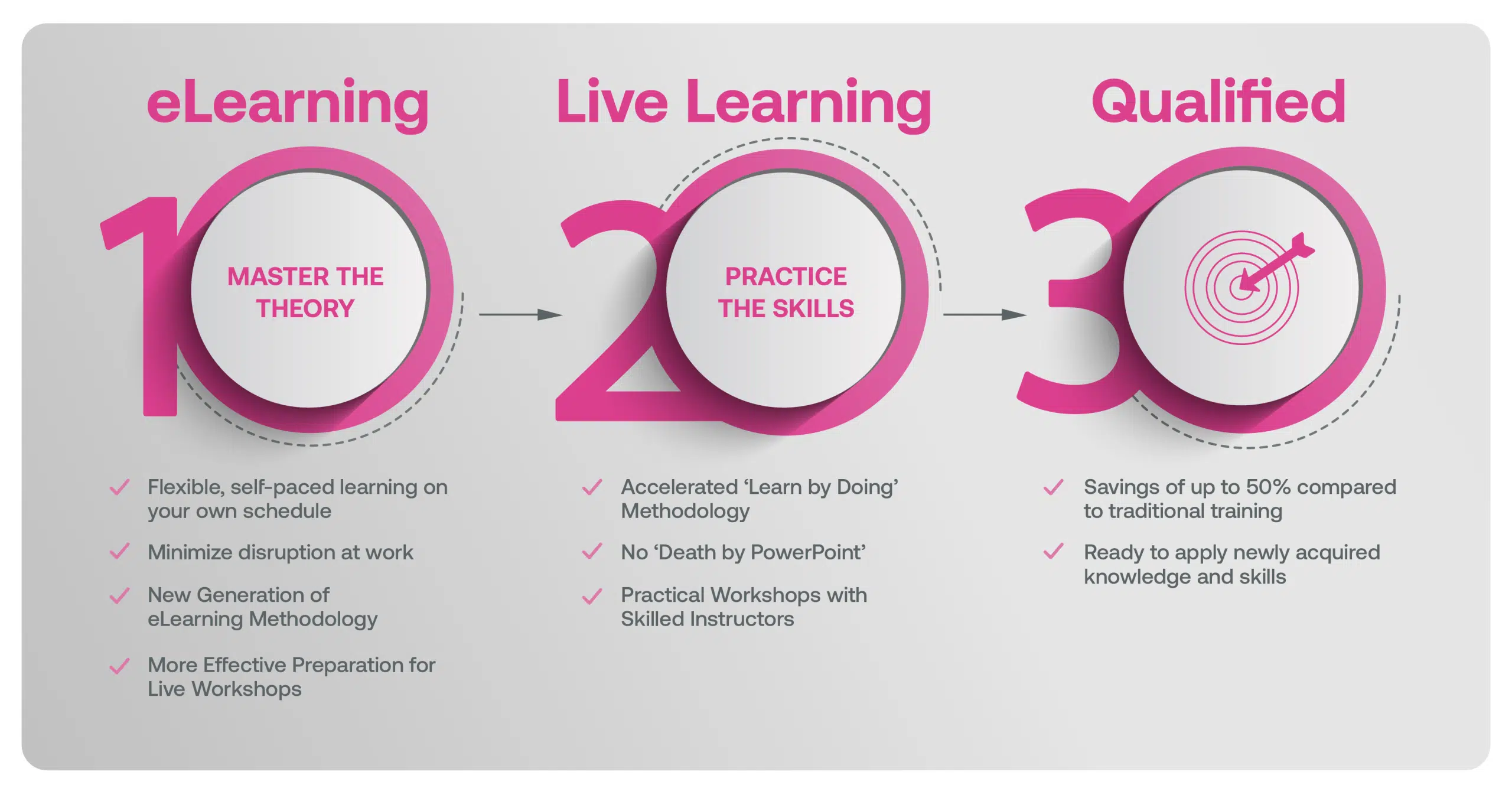
Our blended learning course is a hybrid approach where learners first complete eLearning modules on the underlying theory that better prepares learners in advance of attending Live Classes with an experienced Instructor. Our live workshops are based on practical training methods with real-world examples, case studies, facilitated exercises and group discussions.
CQI and IRCA certify this course (No. 2765) offering global recognition for successful participants.





View Sample Certificate of Achievement


Need Corporate, In-House or Customized Training?
Comply Guru offers corporate, in-house and customized training solutions for your organization’s specific needs.
Speak with an experienced member of our team today to learn how we can help.
Upcoming Schedule
Gain a Globally Recognized Internal Auditor Qualification
Advance your knowledge and enjoy more flexibility & learning effectiveness with Comply Guru.

Register 3 + Get 1 Free

Course Overview
ISO 13485 Internal Auditor Training
Explain the purpose of a medical device quality management system (MD QMS), of medical device quality management systems standards, and the business benefits of improved performance given by an effectively implemented medical device quality management system
Outline the structure and content of ISO 13485 and its relationship with ISO 9001 and IMDRF individual country regulatory documents
Explain the specific quality management-related requirements of ISO 13485
With reference to the PDCA cycle, explain the process-based QMS Model for ISO 13485 and the role of an internal auditor in the maintenance and improvement of Quality Management Systems
Explain the role and responsibilities of an auditor to plan, conduct, report, and follow up an internal audit in accordance with ISO 19011
CQI & IRCA certify this course (No. 2765). Upon successful completion, each Learner shall receive a digital Certificate of Achievement within 1 business day.
Before completing this course, each Learner should have the following prior knowledge:
- An understanding of the requirements of ISO 13485 and the commonly used quality management terms and definitions, which may be gained by completing a CQI and IRCA Certified MD-QMS ISO 13485:2016 Requirements / Foundation (FD132) course or equivalent.
- The Plan, Do Check, Act (PDCA) Cycle
- The core elements of a management system and the interrelationship between top management responsibility, policy, objectives, planning, implementation, measurement, review, and continual improvement.
- The fundamental concepts and the seven quality management principles (see ISO 9000).
- The relationship between quality management, regulatory authority, and customer requirements.
- The ISO 19011 audit process.
- Regulatory authority requirements not directly covered in ISO 13485.
Fluency in written and spoken English
- For participants whose first language is not English, we recommend a minimum English language competency of IELTS 5.5 (or equivalent) for successful completion of the program. This is not assessed by Comply Guru in advance & each participant must self-assess their competency.
Copy of ISO 13485 (International Standard)
- It is recommended that each Learner should have a copy of ISO 13485:2016 (International Standard) to reference while completing this course. This is not provided by Comply Guru and without it, this course will be challenging to complete successfully.
In order to successfully complete this course, each Learner will need to:
- Complete the eLearning modules and obtain 70% or higher in the final assessments (MCQ-based) by the required deadline set in advance of the given workshop dates you are registered for (applies to blended format only)
- Fully attend the Instructor Workshops as 100% attendance is required
- Obtain 70% or higher in the graded assessments during the Instructor Workshops
For the live workshops during a virtual delivery, we utilise both Zoom and Microsoft Teams.
Learners need to individually have:
- PC or MAC Computer
- Reliable Internet
- Video Webcam
- Headset or Earbuds
- Quiet Setting
In relation to the eLearning Modules, in our experience, Workplace IT environments’ internal configurations and available software can vary (new or old), and there may be various limitations or other restrictions in place, and as such, the functionality of any Learning Management System (LMS) may be impacted, restricted and may not perform well. Read the full technology requirements here.
Our Methodology
Blended Learning is Better Learning
A two-step methodology with eLearning modules that help you master the theory better preparing you for practical workshops that embed the skills.
Course Structure Explained
Detailed Breakdown & Agenda
Learners first complete interactive eLearning modules to grasp the underlying theory, then attend live, instructor-led workshops emphasizing practical, real-world application.
| Time | Topic |
|---|---|
| 150mins eLearning | Module 1: Overview of ISO 13485
|
| 120mins eLearning | Module 2: Introduction to Auditing
|
| 40mins eLearning | Module 3: Audit Initiation and Preparation
|
| 80mins eLearning | Module 4: Conducting Audit Activities
|
| 25mins eLearning | Module 5: Audit Report, Close and Follow Up
|
| Time | Topic |
|---|---|
| 09:00 - 10:00am | Course Introduction |
| 10:00 - 10:05am | Break |
| 10:05 - 11:05am | Practical Workshop Audit Checklist |
| 11:05 - 11:15am | Break |
| 11:15am - 12:15pm | Practical Workshop Opening Meeting |
| 12:15 - 12:20pm | Break |
| 12:20 -1:00pm | Practical Workshop Generating Audit Findings |
| 1:00 - 1:45pm | Lunch |
| 1:45 – 2:45pm | Practical Workshop Generating Audit Findings (Continued) |
| 2:45 - 2.50pm | Break |
| 2:50 – 3:50pm | Practical Workshop Closing Meeting, Audit Report & Follow-Up |
| 3:50 - 4:00pm | Break |
| 4:00 - 5.00pm |
|
Our Experts
Meet The Instructors
Our experts possess a wealth of industry experience acquired over years of practical application, and in addition, they demonstrate a combination of unwavering passion and a proven aptitude for training.
Watch and Learn More
About Our Internal Auditor Training
Learn about how our IRCA Accredited Internal Auditor Training is leading the industry for innovation & learning effectiveness
Customer Reviews
What Our Learners Are Saying
Read verified reviews from Learners who have completed this course.
4.6
Average Rating
457 global ratings
-
My overall training experience with Comply Guru was very positive. Michelle Keane was extremely well prepared and delivered the sessions with great knowledge and experience. She explained topics clearly, answered questions confidently, and made the training practical and engaging throughout.
-
eLearning is great as I can do it at my own pace, which is perfect for me.
-
My experience was very positive. Thanks for this opportunity, that I could have taken part in this training.
-
Valuable and clear advice from Michelle. Breaking down the standard made it easier to follow and understand.
-
The training was great with case studies which made it easy to understand the purpose of internal auditing. Tess, our trainer was very knowledgeable, supportive and friendly which made the training effective.
Frequently Asked Questions