Webinars
Show Me All Webinars
- iso 13485
- internal auditing
- medical devices
- eu mdr
- eu ivdr
- prrc
- unannounced audit
- quality
- mdsap
- auditing
- version 9
- fda
- qmsr
- manufacturers
- final rule
- medtech
- training
- open day
- qara
- technical documentation
- regulatory affairs
- post market surveillance
- capa
- fda qmsr
- transition
- gap analysis

Navigating the FDA QMSR Transition: A Practical Gap Analysis Approach
This is a Free Comply Guru Webinar where Navigating the FDA QMSR Transition: A Practical Gap-Analysis Approach will be discussed by Michelle Keane.

How to Prevent Weak CAPA Investigations
This is a Free Comply Guru Webinar where How to Prevent Weak CAPA Investigations will be discussed by Michelle Keane and special guest Jackie Torfin.

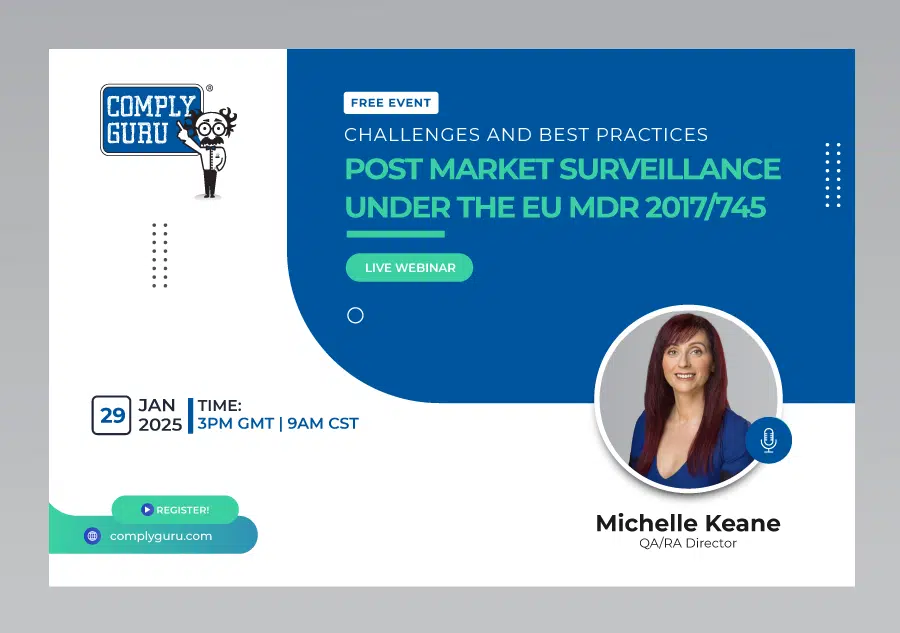
Post Market Surveillance under the EU MDR – Challenges and Best Practices
This is a Free Comply Guru Webinar where Post-Market Surveillance under the EU MDR – Challenges and Best Practices will be discussed by Michelle Keane.

Technical Documentation under the EU MDR 2017/745
This is a Free Comply Guru Webinar where Technical Documentation under the EU MDR will be discussed by Michelle Keane and Orla Keane.

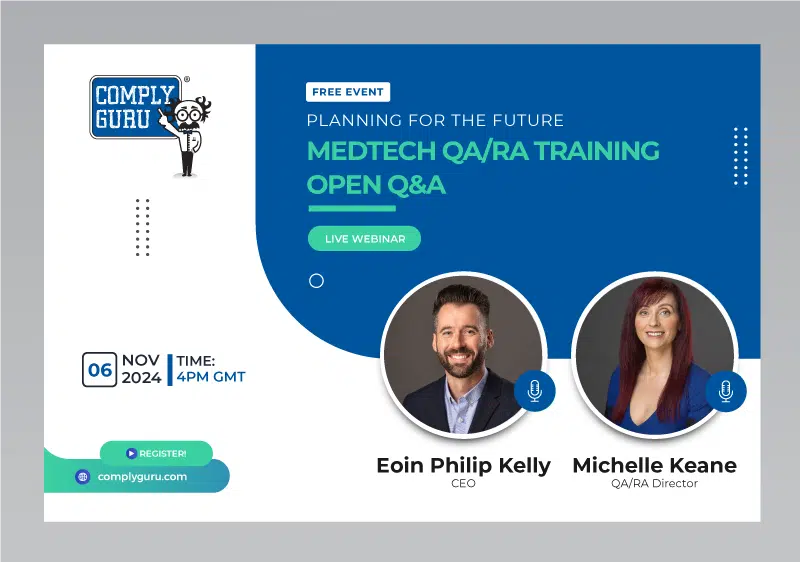
MedTech Quality and Regulatory Affairs (QARA) Training Open Q&A
Planning ahead on training needs? Looking to budget? Join us on Wednesday, November 6th 2024, from 4pm GMT, where Comply Guru’s Eoin P. Kelly & Michelle Keane will discuss Comply Guru’s MedTech Quality and Regulatory Affairs Training Portfolio. It is a great opportunity to come and ask questions about any course!

Impact of the FDA QMSR on Medical Device Manufacturers
This is a Free Comply Guru Webinar where the new FDA QMSR Final Rule and its impact on Manufacturers will be discussed by Michelle Keane.

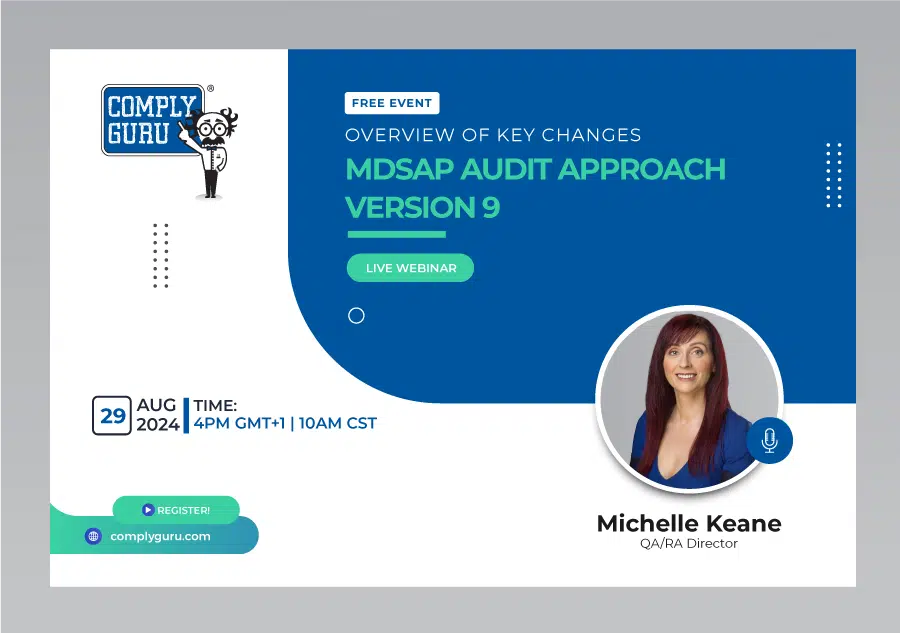
Overview of Key Changes with MDSAP Audit Approach Version 9
This is a Free Comply Guru Webinar covering an Overview of Key Changes with MDSAP Audit Approach Version 9 with Michelle Keane.

Unannounced Audits in the Medical Device Industry
This is a Free Comply Guru Webinar on Unannounced Audits with Michelle Keane.

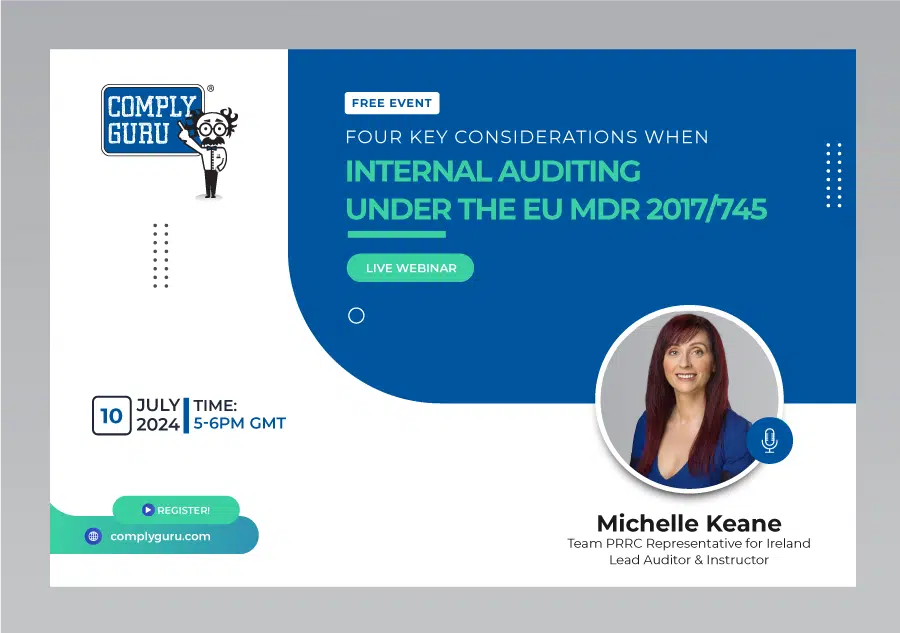
Four Considerations when Internal Auditing under the EU MDR Webinar
This is a Free Comply Guru Webinar on Key Considerations when Internal Auditing under the EU MDR with Michelle Keane.

The Role of the Person Responsible for Regulatory Compliance (PRRC) Webinar
This is a Free Comply Guru Webinar on the Role of the Person Responsible for Regulatory Compliance (PRRC) under the EU MDR and EU IVDR with Michelle Keane.